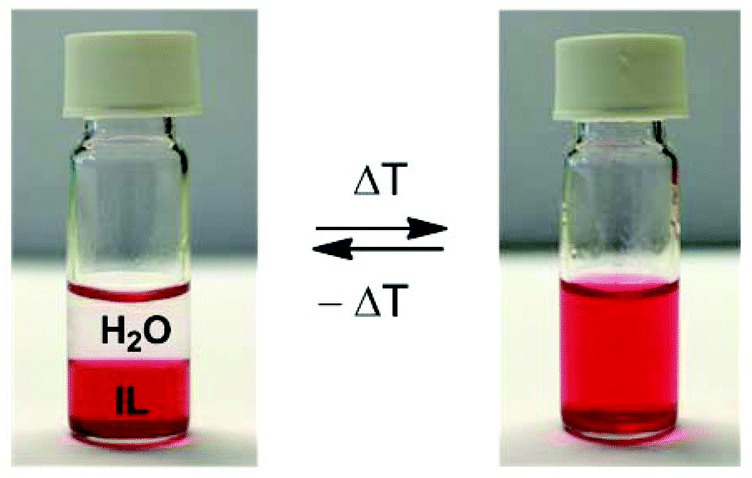
What makes a good solvent for recrystallization is that the compound doesn't dissolve when the solvent is cold but only when it is hot. This is true for benzoic acid
Benzoic acid
Benzoic acid, C₇H₆O₂, is a colorless crystalline solid and a simple aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only known source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many second…
What makes a good solvent for recrystallization?
What makes a good solvent for recrystallization is that the compound doesn't dissolve when the solvent is cold but only when it is hot. This is true for benzoic acid in water.
Why is water a good solvent for dissolving crystals?
Because of its polarity and ability to form hydrogen bonds, water makes an excellent solvent, meaning that it can dissolve many different kinds of molecules. Similarly, why is ice cold solvent used to rinse the purified crystals?
What is recrystallization used for in chemistry?
In chemistry, recrystallization is a technique used to purify chemicals. By dissolving both impurities and a compound in an appropriate solvent, either the desired compound or impurities can be removed from the solution, leaving the other behind. How is recrystallization done?
How much solubility is required for recrystallization?
If it takes more than 3 mL to dissolve the sample in the hot solvent, the solubility in this solvent is probably too low to make it a good recrystallization solvent. Procedure. Why water is not good solvent for the recrystallization?
Is water a good solvent for recrystallization?
Is water a solvent?
Why is water used as a solvent in recrystallization?
A lot of times water is used for recrystallization of organic chemicals because they DON'T want to dissolve in such an extremely polar liquid (and it's so cool that water is so cheap!) but at 100 deg C, the temperature weakens the intermolecular attractions, forcing the organic to fall apart.
What makes a good solvent for recrystallization?
A good recrystallization solvent should (1) dissolve a moderate quantity of the substance being purified at an elevated temperature, but only a small quantity at low temperatures, (2) not react with the substance being purified, (3) dissolve impurities readily at a low temperature or not dissolve them at all, and (4) ...
Why is water a good solvent for recrystallization of benzoic acid?
Data suggests that Water is the best solvent that will allow for better saturation and the best recrystallization of benzoic acid, this is largely due to water being a polar molecule whose properties allow for carboxylic acid groups, such as the one found in benzoic acid, to disassociate and donate protons to the water ...
Why is water used as recrystallization solvent for Acetanilide?
The crude solid is dissolved in the smallest possible amount of solvent of choice; in this case the solvent is water. Acetanilide has a much higher solubility in hot water than in cold water. The purified solid will not recrystallize later in the experiment if too much hot solvent is added in the beginning.
What are the characteristics of a good solvent?
Not toxic, not flammable.Immiscible pair of solvents: water and low polarity organic solvents. ... Good solubility of the target compound. ... Poor solubility of impurities. ... Volatility of the extraction solvent. ... Toxicity and safety properties of the extraction solvent.
What properties should a solvent have to be a good recrystallization solvent for a particular compound under what conditions is a mixed solvent appropriate?
For mixed solvent recrystallization your material should be relatively soluble in one solvent and relatively insoluble in another solvent. For example, a substance which is very soluble in alcohol and almost insoluble in water may crystallize well from a mixture.
Why is ethanol a better solvent than water for recrystallization?
Ethanol/water combinations are commonly used because ethanol has good dissolving ability for many organics, but is also infinitely co-soluble with water. Addition of water can rapidly and dramatically reduce the solubility of many organics and thus induce crystallization.
What advantages and disadvantages does water have as a recrystallization solvent?
Water. Answer: Advantage is its low cost and low toxicity; disadvantage is the difficulty of removing it from products due to low volatility.
Which solvent is most suitable for the recrystallization of acetanilide?
Acetanilide is readily soluble in ethanol at room temperature. So we can not use the ethanol as a solvent for acetanilide recrystallization. But it can soluble in water when heating. Therefore, water is the beat solvent for acetanilide.Oct 24, 2014
Why is it important to use a minimum amount of solvent for recrystallization?
The quantity of solvent used in crystallization is usually kept to a minimum, to support the goal of recovering the maximum amount of crystals. Every solid has partial solubility in the solvents used, even at cold temperatures.Apr 7, 2022
Which solvent is used for recrystallization of benzoic acid?
hot waterRecrystallization: Using a hot plate, dissolve approximately 1.0 g of impure benzoic acid in 30 – 35 mL of hot water (water at or near its b.p.) in a 125 mL Erlenmeyer flask.
Help! Recrystallization sources of error. : chemhelp
The purpose of the lab was to purify Phthalic acid through recrystallization. We used water as the solvent. Place acid into water and bring to boil …
(PDF) CHEM 2423 Recrystallization of Benzoic Acid EXPERIMENT 4 ...
CHEM 2423 Recrystallization of Benzoic Acid EXPERIMENT 4 -Purification -Recrystallization of Benzoic acid
experimental chemistry - Why is the solid product washed with ice-cold ...
$\begingroup$ Recrystallisation may be done in a multitude of ways, depending on your particular situation; the product may be washed with ice-cold solvent, or with another solvent that would supposedly dissolve the impurities but not the product, or not washed at all. You may call it weird, but then again, pretty much all chemistry is weird in the same sense, or worse.
Melting Point and Recrystallization - PHDessay.com
Essay on Melting Point and Recrystallization Recrystallization was done to remove impurities from the sample. The percent recovery of benzoic acid during recrystallization is 23. 02%. The difference
What are the characteristics of a good recrystallization solvent?
Characteristics of a Good Recrystallization Solvent: The recrystallization solvent should NOT dissolve the substance to be purified at room temperature, but it should dissolve it well at the solvent's boiling point 2. The solvent should dissolve soluble impurities well at room temperature. Click to see full answer.
Why is water a good solvent?
Because of its polarity and ability to form hydrogen bonds, water makes an excellent solvent, meaning that it can dissolve many different kinds of molecules.
What solvent do you use to clean crystals?
When vacuum filtering, wash your crystals with the solvent you used to recrystallize your compound. However, use ice-cold solvent to ensure that you do not dissolve any of your crystals.
Can a solvent dissolve at room temperature?
The solvent must not dissolve the compound at low temperatures (that includes room temperature), but must dissolve the compound at high temperatures. What is an example of a solvent? Solvent is, in simplest terms, something in which you dissolve another substance (also called as solute) and this mixture will yield what we know as a 'solution'.
Is water a good solvent for recrystallization?
For most organic compounds, water is not a good recrystallization solvent. Crystals (i.e., the pure compound) should form in the flask and leave the soluble impurities behind in the solution.
Is water a solvent?
Water is a good solvent for re-crystallization of acetanilide, because the solubility of acetanilide is about 1g in 20mL of boiling water, but only 1g of acetanilide per 185 mL ofcold water. Thus crystallizes when cooled. Click to see full answer.