Fractional distillation is a method for separating liquids with different boiling points. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points.
How do you separate a mixture by fractional distillation?
Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points.
What is an example of fractional distillation?
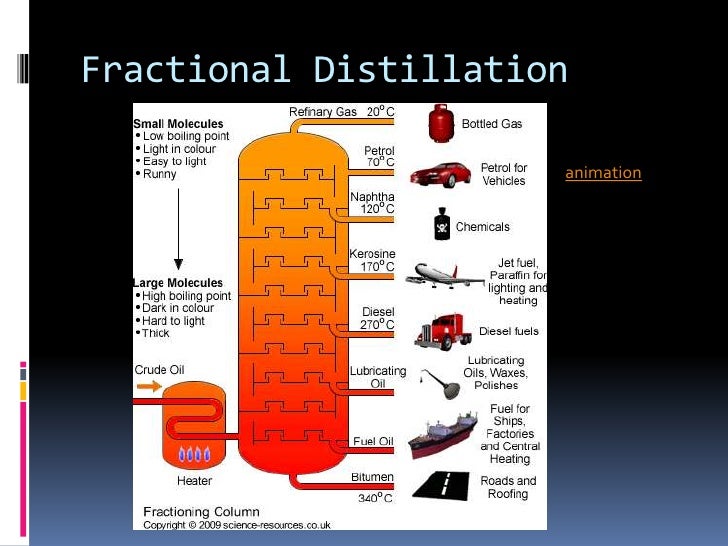
Fractional Distillation of Crude Oil. A common example of fractional distillation in industries is the separation of various components of crude oil. Crude oil normally contains substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil and kerosene.
What is the fractional method of separation?
The Fractional method takes advantage of the different boiling points of the two liquids. Example: To separate ethanol from a solution of water and ethanol, it would work like this:
How do you separate a mixture of ethanol and water?
For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points.
Which liquids are distilled using the fractional distillation method?
Those liquids with nearly identical boiling points, indicating that their boiling point is not very high. In this method, such liquids are used.
Identify the two critical conditions for fractional distillation.
The conditions for fractional distillation are- The liquids must be miscible with each other. The difference between the boiling points of the two...
What are the steps involved in Fractional distillation?
The steps of the process are: Evaporation Condensation Collection
What is the difference between simple and fractional distillation?
Simple distillation is used to separate substances in mixtures with widely disparate boiling points, whereas fractional distillation is used for mi...
What is the principle of fractional distillation?
Different liquids boil and evaporate at different temperatures, which is the basic principle of this type of distillation. As a result, when the mi...
What is fractional distillation?
Similar to simple distillation, fractional distillation is best for separating a solution of two miscible liquids. (Miscible liquids are liquids that dissolve in each other). The Fractional method takes advantage of the different boiling points of the two liquids. Example: To separate ethanol from a solution of water and ethanol, ...
How does a fractional distillation column work?
The column helps the rising gas to slowly condense and re-evaporate several times before it is collected into the beaker. In the end, the water is separated from the ethanol. Fractional distillation takes a bit more time than simple distillation.
What happens when you mix ethanol and water?
In a mixture of two miscible liquids (like water and ethanol), one liquid will have a lower boiling point (ethanol). In a similar set-up like a simple distillation, the heat is applied to the solution to raise its temperature to the boiling point of the ethanol. That will turn the ethanol in the mixture into a gas.
What is fractional distillation?
Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points.
How does ethanol work?
When the mixture is heated, one liquid evaporates before the other. A water and ethanol mixture is heated in a flask using an electric heater. Vapour forms in the air above the mixture in the flask. The boiling point of ethanol is 78°C.
Why does fractional distillation work?
This method works because the liquids in the mixture have different boiling points. When the mixture is heated, one liquid evaporates before the other. In this experiment, we will use fractional distillation to separate a mixture of ethanol and water.
How does ethanol get cooled?
The ethanol vapour passes into the condenser, gets cooled and collects in a beaker kept at the other end of the condenser. The temperature is maintained at 78°C until no more liquid is collected in the beaker. (Note that very little water distills over because 78°C is less that the boiling point of water.