What is the equation for magnesium react with oxygen?
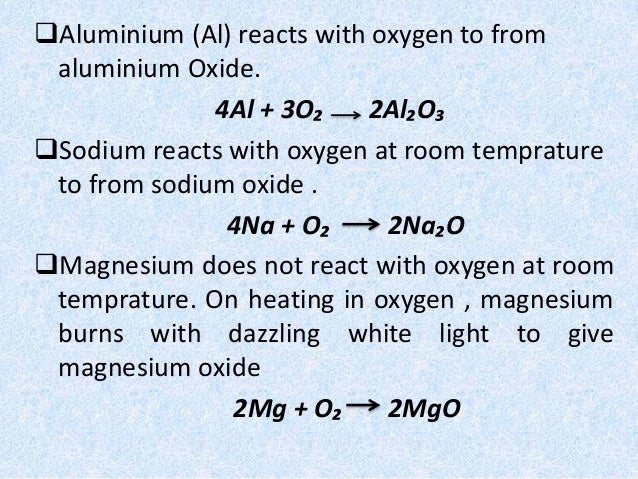
Jan 30, 2020 · The formula for magnesium oxide is MgO. Mg donates two electrons to become the Mg+2 ion and O accepts two electrons to become the O-2 ion. The reaction between Mg and O2 is written as follows: 2 Mg (solid) + O2 (gas) -> 2 MgO (solid).
What is the word equation for magnesium and oxygen?
Feb 12, 2020 · Magnesium, I guess, means elemental magnesium - which is typically just a metal. This means that it's possible for single atoms to react, so the formula is Mg_1, or just Mg. Oxygen is commonly a diatomic gas, meaning that it contains 2 atoms per molecule - hence a formula of O_2. Mg + O_2 -> MgO.
Is magnesium oxygen and magnesium oxide the same thing?
Oct 23, 2012 · What is the word equation for the reaction of magnesium with oxygen? WORD EQUATION: Magnesium + Oxygen --> Magnesium oxide SYMBOLS: 2Mg + O2 --> 2MgO
What is the chemical formula for magnesium plus oxygen?
Word equation. magnesium + oxygen → magnesium oxide. Chemical equation. 2 Mg + O 2 → 2 MgO. Picture equation
What is the equation for magnesium and oxygen?
ExampleStepResult1magnesium + oxygen → magnesium oxide2Mg + O 2 → MgO3reactants: 1 × Mg, 2 × O products: 1 × Mg, 1 × O Not balanced.4Mg + O 2 → 2MgO3 more rows
What is the word equation for magnesium chlorine oxygen?
MgCl2+3O2→Mg(ClO3)2.
How do you write the equation for magnesium oxide?
Expert Answer:Balancing an equation.Let us first write the word equation for this reaction. Magnesium + Oxygen → Magnesium oxide. ... Write the chemical equation for the reaction between magnesium and oxygen. Mg + O2 → MgO.More items...•Jan 18, 2021
What is the word equation for magnesium and water?
Equation 5: Mg(s) + 2H2O(g) → Mg(OH)2 (aq) + H2(g)Nov 9, 2020
What is the chemical formula for magnesium?
MgQuestion Video: Writing the Chemical Formula of the Magnesium Ion Chemistry • 7th Grade. A magnesium atom has the chemical formula Mg.Sep 6, 2021
What is the equation of magnesium chloride?
MgCl2Magnesium chloride / Formula
What is the formula of magnesium nitride?
Mg₃N₂Magnesium nitride / Formula
What is the formula of magnesium sulphate?
MgSO₄Magnesium sulfate / FormulaThe chemical formulae for magnesium sulfate are MgSO4 (anhydrous) and MgSO4. xH2O (hydrated forms), and the molecular weights are 120.36 (anhydrous), 138.38 (monohydrate), and 246.47 (heptahydrate).
What is the word equation for magnesium and calcium?
Calcium magnesium oxidePubChem CID161939Molecular FormulaCaMgO2SynonymsCalcium magnesium oxide 37247-91-9 Dolomitic lime Calcum magnesium oxide EINECS 253-425-0 More...Molecular Weight96.38Component CompoundsCID 5462224 (Magnesium) CID 190217 (Oxide) CID 5460341 (Calcium)3 more rows
What is magnesium and oxygen?
When magnesium reacts with oxygen, it produces light bright enough to blind you temporarily. Magnesium burns so bright because the reaction releases a lot of heat. As a result of this exothermic reaction, magnesium gives two electrons to oxygen, forming powdery magnesium oxide (MgO).Apr 12, 2019
What does a word equation show?
Key points. A word equation represents a chemical reaction using the names of the substances involved. Word equations do not show any chemical symbols or formulae . In a chemical reaction, reactants are the substances that react together, and products are the substances formed.
What are the two groups of metals?
Any two of the following: BeO, MgO, CaO, SeO, BaO. Group 1 metals are referred to as the Alkali Metals and Group 2 metals are referred to as the Alkaline Earth Metals. Iron is from Group 8. Here is the picture equation of the reaction between iron and oxygen (iron is green and oxygen is red).
What is the color of iron oxide?
It is a browny, red colour. Rust is a word to describe the flaky, crusty, reddish-brown product that forms on iron when it reacts with oxygen in the air. When your teacher burned the iron earlier, it reacted quickly with oxygen to form iron oxide. Here is a picture of iron oxide to remind you what it looked like.
What is the reaction of magnesium and oxygen?
The reaction of magnesium with oxygen. It is recommended that you demonstrate the reaction to the learners, because of the hazards involved with burning metals. Instructions: Wear safety goggles and a protective coat. Caution learners not to look directly at the intense white flame produced by the burning magnesium.
What is the process of coating iron with zinc?
Iron can be coated with a thin layer of zinc in a process called galvanising .The zinc layer quickly reacts with oxygen to become zinc oxide. This layer protects the zinc underneath it from being further oxidised. It also protects the iron underneath the zinc from contact with oxygen.
Why is iron not protected?
So even though the iron surface is covered, it is not protected, because the oxygen molecules can still reach the iron to react with it. There are a number of other ways to stop or slow down rust. One way to protect the iron surface is to cover it with a metal that does not corrode, like chromium, for instance.
Why is it important to protect taps from rust?
This is because they are in a moist, humid environment and water makes iron more prone to rust.
What are the elements in steel wool?
NOTE: The other elements in steel include carbon, manganese, phosphorus, sulfur, silicon, and traces of oxygen, nitrogen and aluminum. Learners do not need to know the names of the other elements in steel wool. Look at the metal before it is burned.
What is the reaction of magnesium and oxygen?
When the magnesium metal burns it reacts with oxygen found in the air to form Magnesium Oxide. A compound is a material in which atoms of different elements are bonded to one another. Oxygen and magnesium combine in a chemical reaction to form this compound.
What is a word equation?
In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. The words "and" or "plus" mean one chemical and another are both reactants or products. The phrase "is reacted with" indicates the chemicals are reactants.
What are the types of chemical reactions?
The main four types of reactions are direct combination, analysis reaction, single displacement, and double displacement. If you're asked the five main types of reactions, it is these four and then either acid-base or redox (depending who you ask).
What is the formula for elemental oxygen?
The element, oxygen, can exist in several forms, as atomic oxygen (O), or as the dimer oxygen (O2), or as the trimer ozone (O3).
Why is mg o2 MgO?
Magnesium (Mg) is a divalent metal, which means that its cation has a +2 charge. Oxygen (O) is also divalent for its ion is -2 charged. So, to answer your question, the 2 from O reacts with another atom of Mg to form more MgO. Why is oxygen in chemical equations written as O2, when the element itself is just O?
How do you balance equations?
To balance a chemical equation, start by writing down the number of atoms in each element, which is listed in the subscript next to each atom. Then, add coefficients to the atoms on each side of the equation to balance them with the same atoms on the other side.
What is the word equation for burning magnesium?
The increase in mass is due to the fact that oxygen from the air has combined with the magnesium to make magnesium oxide, MgO. The chemical equation, Mg + O2 MgO shows this reaction but it needs to be balanced to make 2Mg + O2 2MgO.
Which side of the equation is magnesium oxide?
The magnesium oxide is formed that is it is product in the reaction so it is written on the right side of the . Hence, the word equation for molecules of magnesium reacting with molecules of oxygen to produce molecules ...
What side of the equation is the reaction?
In order to write the word equation for a reaction, the reactants are always written on left side of the arrow, and products are written on the right side. sign is used to show the addition of two or more elements or compounds in reactants side to form product. One or more product can form during a reaction and are separated by sign.