Is an excited state more stable than a ground state?
The ground state is the state where electrons contain the lowest energy in an atom and tend to be highly stable, but the excited state is the state where electrons have higher energy than ground state and have lower stability.
Which of electron configurations represent an excited state?
Which of the following electron configurations represents an excited state of the indicated atom? Which of the following electron configurations, I-IV, represents an excited state? I. [He]2s12p5 II. [Kr] 5s24d105p1 III.
What is an example of an excited state?
Long-lived excited states are often called metastable. Long-lived nuclear isomers and singlet oxygen are two examples of this. A simple example of this concept comes by considering the hydrogen atom .
What is ground state and excited state?
ground state (of a quantum-mechanical system): the lowest energy state of the system. Excited state: any state with energy greater than the ground state. Please, note that the ground state is only about its energy level. There may exist more than one ground state for some quantum-mechanical systems.
What is excited state state?
Definition of excited state : a state of a physical system (such as an atomic nucleus, an atom, or a molecule) that is higher in energy than the ground state.
How do you know if an electron configuration is in an excited state?
0:224:37Excited state means that one or more electron was excited or bumped up to a higher level so theMoreExcited state means that one or more electron was excited or bumped up to a higher level so the lower levels weren't completely filled up. Before you had you filled into higher levels.
What does excited state mean in chemistry?
When an electron temporarily occupies an energy state greater than its ground state, it is in an excited state. An electron can become excited if it is given extra energy, such as if it absorbs a photon, or packet, of light, or collides with a nearby atom or particle.
What is an excited state configuration for oxygen?
The excited-state electron configuration for Oxygen is 1s22s22p33s1.
What is excited state and ground state?
Ground state means the lowest energy state. When the electrons absorb energy and jump to outer orbits, this state is called excited state.
Which electron configuration notation describes an atom in an excited state?
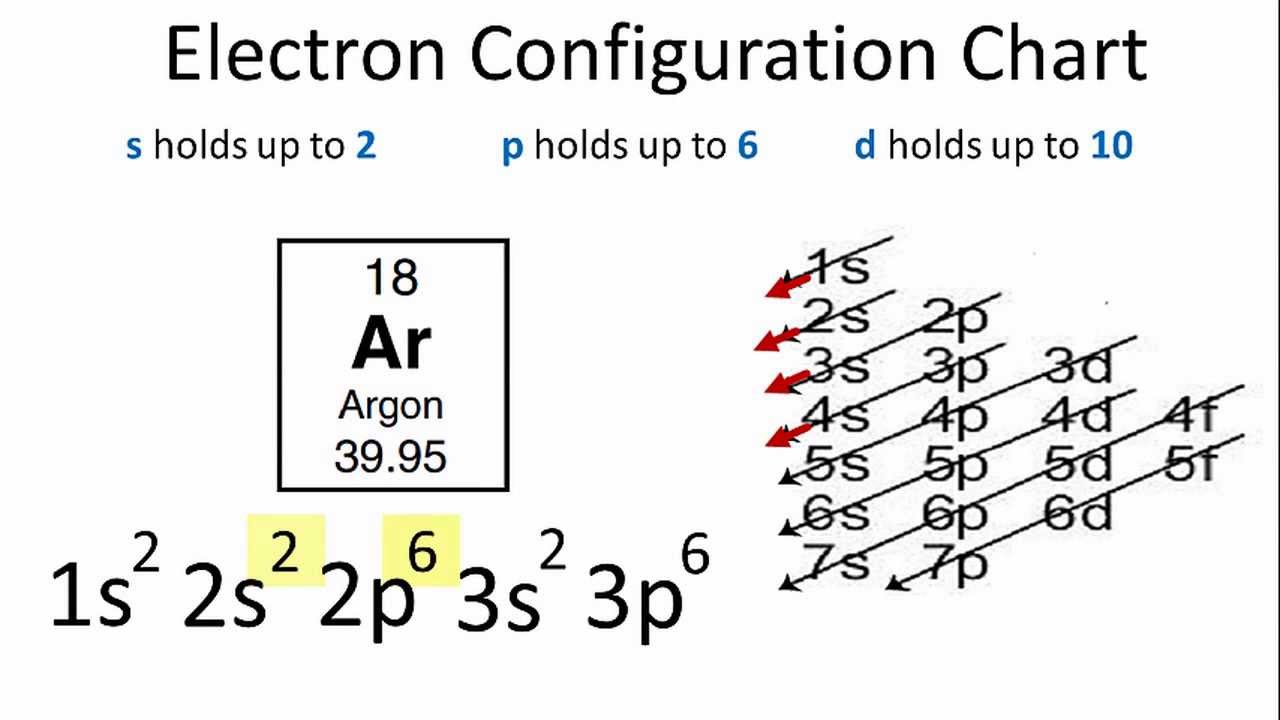
Explanation: An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. The ground state electron configuration of sodium is 1s22s22p63s1 .
What is excited state of a molecule?
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum).
What is an excited state quizlet?
Excited state is any state of an atom that is higher than the ground state. Energy has to be applied to the atom for the electrons from electromagnetic radiation(light) or from collisions with other particles.
What is the excited state electron configuration of carbon?
1s2 2s1 2p3The ground state electron configuration of carbon is 1s2 2s2 2p2. An excited state electron configuration of carbon is 1s2 2s1 2p3.
How many excited states does oxygen have?
two excited statesThis more stable of the two excited states has its two valence electrons spin-paired in one π* orbital while the second π* orbital is empty. This state is referred to by the title term, singlet oxygen, commonly abbreviated 1O2, to distinguish it from the triplet ground state molecule, 3O2. ) ground states.
What is the electron configuration for o2 −?
[He] 2s² 2p⁴Oxygen / Electron configuration
What is the correct definition of excited state?
When an atom gains energy in the form of heat or light, the energy can cause some of the electrons to gain energy and jump up to higher orbitals. W...
What is an example of excited state?
One example of the excited state is in photosynthesis in which light energy excites an electron in chlorophyll. This reaction is the beginning of a...
What happens to an excited atom?
If an atom has electrons that are in the excited state it is unstable. The electrons cannot stay there for long and they will go back down to the g...
Violations of the aufbau principle
Let's take a look at an example that violates the aufbau principle. The electron configuration of an atom is shown below:
Try it!
Hund's rule violations also result in higher energy electron configurations and therefore are excited states. The figure below (a) shows that if an electron is paired when other degenerate orbitals are empty, this will be of higher energy (increased electron repulsion).
What is an excited state?
Updated April 10, 2019. The excited state describes an atom, ion or molecule with an electron in a higher than normal energy level than its ground state . The length of time a particle spends in the excited state before falling to a lower energy state varies.
What are some examples of metastable states?
Examples of metastable states are single oxygen and nuclear isomers. Sometimes the transition to an excited state enables an atom to participate in a chemical reaction. This is the basis for the field of photochemistry.
Table of Contents
Excited State of an Atom: What does it Mean When the Electrons are Excited?
Excited State Definition Chemistry
Atoms are made up of protons, neutrons, and electrons. The protons are positively charged and the neutrons are neutral. These are both found in the nucleus which causes the nucleus to be positively charged. The other subatomic particle is the electron which is negative. A neutral atom contains the same number of electrons as protons.
Excited State of an Atom: What does it Mean When the Electrons are Excited?
When an atom absorbs heat or light that energy has to go somewhere. That energy goes into the electrons which are virtually massless and easy to move. The electrons can only move to certain orbitals with specific amounts of energy, so they will only absorb a certain amount of energy.
What is the first excited state?
The one closest to the nucleus is called the ‘first excited state’ (lowest energy), whereas, the second farther is called the ‘second excited state’, and so on. Consider Phosphorous, a chemical element with symbol ‘P’. Its atomic number is 15. Ground State Electron Configuration : 1s22s22p63s23p3.
What is the excited state of an atom?
Excited State of an Atom: A Definitive Analysis. The smallest bit of a chemical element is termed as an atom. Quantum physics is the branch which explains the structural formation as well as the behavior of an atom. An atom is made up of three particles: electron, proton, and neutron.