How can you tell if a molecule is polar or nonpolar?
How to Determine if a Molecule is Polar Or Nonpolar
- Start by drawing its Lewis structure. This rule applies to all molecules except hydrocarbons and molecules with two atoms of the same element.
- The Lewis structure will help you analyze the shape of the molecule given to you
- Determine which of the five categories of shapes your molecule falls into linear, tetrahedral, trigonal planar, bent, trigonal pyramid. ...
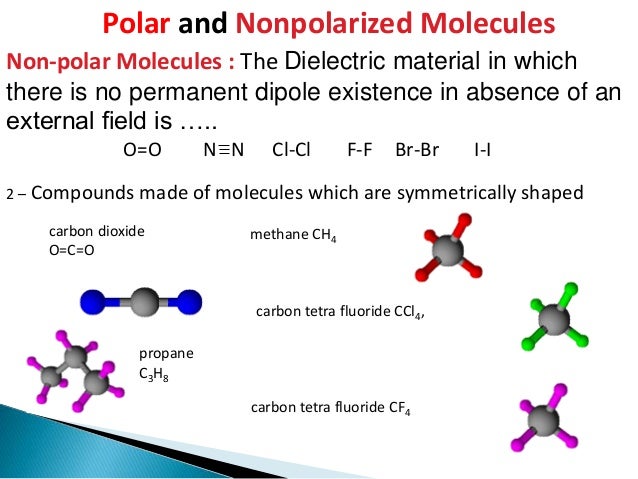
- As learned before, non-polar molecules are perfectly symmetrical while polar molecules are not. ...
- Remember that asymmetry applies even if the outer atoms are the same. The arrangement of the atoms matters more.
- Now, let’s dissect the symmetric molecules. All the atoms that are attached to the central atom must be the same if it is a nonpolar molecule. ...
What makes H20 a polar molecule?
- Uneven distribution of electrons
- Unequal electron charge density
- Uneven size of electron cloud
What is difference between polar and nonpolar compounds?
To summarize, to be polar, a molecule must:
- Draw the Lewis structure.
- Figure out the geometry (using VSEPR theory)
- Visualize or draw the geometry.
- Find the net dipole moment (you don’t have to actually do calculations if you can visualize it)
- If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
What is the difference between polar and non polar molecules?
• Polar molecules have an electrical dipole moment whereas nonpolar molecules don’t have a dipole moment. • Polar molecules have a charge separation in contrast to nonpolar molecules. • Polar substances tend to interact with other polar substances; they don’t like to interact with nonpolar substances.
Is H2O polar or nonpolar and why?
Why is H2O a polar molecule?
What if water is nonpolar?
Why is water considered a polar molecule quizlet?
Why is H2O polar?
Water (H2O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding. When solutes are added to water, they may be affected by the charge distribution.
How to determine if a molecule is polar or nonpolar?
The shape of a molecule and the polarity of its bonds determine the OVERALL POLARITY of that molecule. A molecule that contains polar bonds, might not have any overall polarity, depending upon its shape. The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. If these centers lie at the same point in space, then the molecule has no overall polarity (and is non polar). If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule nonpolar. A molecule can only be polar if the structure of that molecule is not symmetric.
How to determine polarity of a molecule?
The shape of a molecule and the polarity of its bonds determine the OVERALL POLARITY of that molecule. A molecule that contains polar bonds, might not have any overall polarity, depending upon its shape. The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. If these centers lie at the same point in space, then the molecule has no overall polarity (and is non polar). If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule
How many dipoles does CO2 have?
Think of this, it is linear, it 4 dipoles from the Oxygen atoms but, the carbon atom is able to balance out the polarity from those four dipoles. If you tried to run electricity through a chain of CO2 molecules, you would fail. Also, CO2 is not magnetic.
What is a nonpolar covalent bond?
Bonds that are partly ionic are called polar covalent bonds. Nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. The result is a bond where the electron pair is displaced toward the more electronegative atom.
Why is the shape of a molecule not linear?
The reason the shape of the molecule isn't linear and nonpolar (e.g., like CO2) is because of the difference in electronegativity between hydrogen and oxygen. The electronegativity value of hydrogen is 2.1, while the electronegativi
What does polarity mean in chemistry?
polarity = number of electrons that are not matched to the main pain part of the atom. There are several elements this occur in, but most likely, it will be in an element that has a positive or negative charge. In compounds it will happen when all of the electrons are matched to the elements in the compound.
Introduction
Water is one of the most important substances on our planet due to its unique polar chemical arrangement.
The Importance of Polarity
A polar substance is one that has both a positive pole and a negative pole.
The Charges of Atoms
We have to remember that every atom on the periodic table of elements has positive and negative components.
What is Electronegativity?
Electronegativity refers to how strongly an atom attracts another atom’s electrons.
The polarity of water is very important in many areas of life
Because it is able to dissolve so many substances, it has the ability to transfer and distribute useful nutrients and filter out unneeded wastes.
What determines the polarity of a molecule?
The polarity of any molecule depends on these following factors: Its shape. the difference of electronegativities between the atom and. the net dipole moment in the molecule. Here Oxygen atom has the electronegativity of 3.44 whereas the Hydrogen has an electronegativity of 2.20. If you calculate the differences between Oxygen ...
What is the difference between oxygen and hydrogen?
Here Oxygen atom has the electronegativity of 3.44 whereas the Hydrogen has an electronegativity of 2.20. If you calculate the differences between Oxygen and Hydrogen’s electronegativities, it is much higher than 0.5, making the O-H bond a polar covalent bond.
What direction does the dipole moment go?
As a result of this pull, the dipole moment’s direction will be from Hydrogen towards the Oxygen atom on both sides . As a result of the dipole moment on both sides, there is a net dipole moment in this molecule. This would have been avoided if the shape of the molecule was linear.
Why is water a unique molecule?
This happens because the charges near the Oxygen atom are slightly negative and is drawn towards the positive-charged regions of the elements. Similarly, the areas around Hydrogen atoms are positively charged and are attracted to the negatively-charged parts of the molecule.
Why do both hydrogen atoms take up a position like the one shown in the figure?
Both the Hydrogen atoms take up a position like the one shown in the figure to keep the repulsive forces at the minimum. Due to this arrangement of both Hydrogen atoms the shape of the molecule is bent making the geometry of this molecule nonlinear.
Why are the charges near the oxygen atom slightly negative?
This happens because the charges near the Oxygen atom are slightly negative and is drawn towards the positive-charged regions of the elements. Similarly, the areas around Hydrogen atoms are positively charged and are attracted to the negatively-charged parts of the molecule.
How many protons does water have?
Although there are poles in the molecules and a net dipole moment, it is an electrically neutral molecule. Each water molecule is made up of 10 protons, 10 electrons and a zero net charge.
HCl is polar or nonpolar?
HCl is a Hydrochloric acid is an inorganic compound in chemistry. The appearance of HCl is colorless, and it is used in laboratories for many reasons. We cannot bear the smell of HCl for a long time because it is a pungent smell. Many students are confused about if HCl is polar or nonpolar.
Why is HCl polar in chemistry?
HCl consists of two different atoms, so there are different electronegativity values. Here, chlorine is more electronegative in it to attract more to itself. Chlorine gets a negative charge from its negative pole, while hydrogen receives a positive charge from its positive pole.
Conclusion
HCl is a Hydrochloric acid is an inorganic combination in chemistry that is very necessary. HCl is the polar molecule in chemistry. Two charge formations will go on there. In HCl, chlorine is highly collated to a hydrogen atom. Chlorine is near getting a partial negative charge, while hydrogen will gain a partial positive charge.
What determines the polarity of a molecule?
The polarity of any molecule largely depends upon the electronegativities of the atoms forming bonds in the molecule. The most electronegative atom always tries and pulls the shared valence electrons towards itself.
What is the formal charge of H2O2?
Hence the formal charges on the central atom are zero, and the formal charge for H2O2 is also zero.
How many valence electrons are in hydrogen peroxide?
For calculating the formal charges for Hydrogen Peroxide, you can use our simple calculator or use this formula where: For Oxygen, there are six valence electrons, four nonbonded pairs of electrons and four bonding pairs of electrons. Hence the formal charges on the central atom are zero, and the formal charge for H2O2 is also zero.
Which atom is the most electronegative?
For H2O2, Oxygen atoms are the most electronegative ones as each Oxygen atom has an electronegativity value of 3.45. Whereas for the Hydrogen atom, it is around 2.20. The difference of electronegativities for Hydrogen and Oxygen atoms is relatively high, making the H-O bond polar.
What is hydrogen peroxide used for?
Hydrogen peroxide is used as a potent oxidizing and bleaching agent. It is also used as an antiseptic as it can help heal wounds, cuts, etc. H2O2 is used in the production and synthesis of many chemicals. It is also used as a disinfectant and as a sanitizing product.
Is hydrogen peroxide polar or nonpolar?
Hydrogen Peroxide is a polar molecule because there is a net dipole moment in the molecule due to its bent shape. As this molecule has two partially charged molecules, it is polar in nature.
Is H2O2 a polar or nonpolar molecule?
Molecular Shape. H2O2 molecule is a nonplanar and asymmetric molecule. It has a bent geometry due to the presence of two lone pairs of electrons on each Oxygen atom. Due to which the dipole moments in the molecule are not cancelled out. And hence there is a net dipole moment in the molecule, making H2O2 a polar molecule.
Why does a polar molecule have a higher boiling point than a nonpolar molecule?
When comparing a polar and nonpolar molecule with similar molar masses, the polar molecule in general has a higher boiling point, because the dipole–dipole interaction between polar molecules results in stronger intermolecular attractions. One common form of polar interaction is the hydrogen bond, which is also known as the H-bond. For example, water forms H-bonds and has a molar mass M = 18 and a boiling point of +100 °C, compared to nonpolar methane with M = 16 and a boiling point of –161 °C.
Why do polar molecules have polar bonds?
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have a geometry which is asymmetric in at least one direction, so that the bond dipoles do not cancel each other.
What is the difference between polarity and polarity?
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
How to determine polarity of a bond?
Bond polarity is typically divided into three groups that are loosely based on the difference in electronegativity between the two bonded atoms. According to the Pauling scale : 1 Nonpolar bonds generally occur when the difference in electronegativity between the two atoms is less than 0.5 2 Polar bonds generally occur when the difference in electronegativity between the two atoms is roughly between 0.5 and 2.0 3 Ionic bonds generally occur when the difference in electronegativity between the two atoms is greater than 2.0
What is a completely polar bond?
A completely polar bond is more correctly called an ionic bond, and occurs when the difference between electronegativities is large enough that one atom actually takes an electron from the other. The terms "polar" and "nonpolar" are usually applied to covalent bonds, that is, bonds where the polarity is not complete.
Why is it important to determine the point group of a molecule?
Determining the point group is a useful way to predict polarity of a molecule. In general, a molecule will not possess dipole moment if the individual bond dipole moments of the molecule cancel each other out. This is because dipole moments are euclidean vector quantities with magnitude and direction, and a two equal vectors who oppose each other will cancel out.
How many groups are there in bond polarity?
Bond polarity is typically divided into three groups that are loosely based on the difference in electronegativity between the two bonded atoms. According to the Pauling scale :
Introduction
The Importance of Polarity
The Charges of Atoms
What Is electronegativity?
The Polarity of Water
- As you may know, the chemical symbol for water is H2O. This means that it has two hydrogen atoms and one oxygen atom. The oxygen atom is negatively charged, and the two hydrogen atoms bonded to it are positive. These atoms are arranged so that the oxygen atom is in the middle, with the two hydrogen atoms near each other, but not touching, so it loo...
The Polarity of Water Is Very Important in Many Areas of Life.