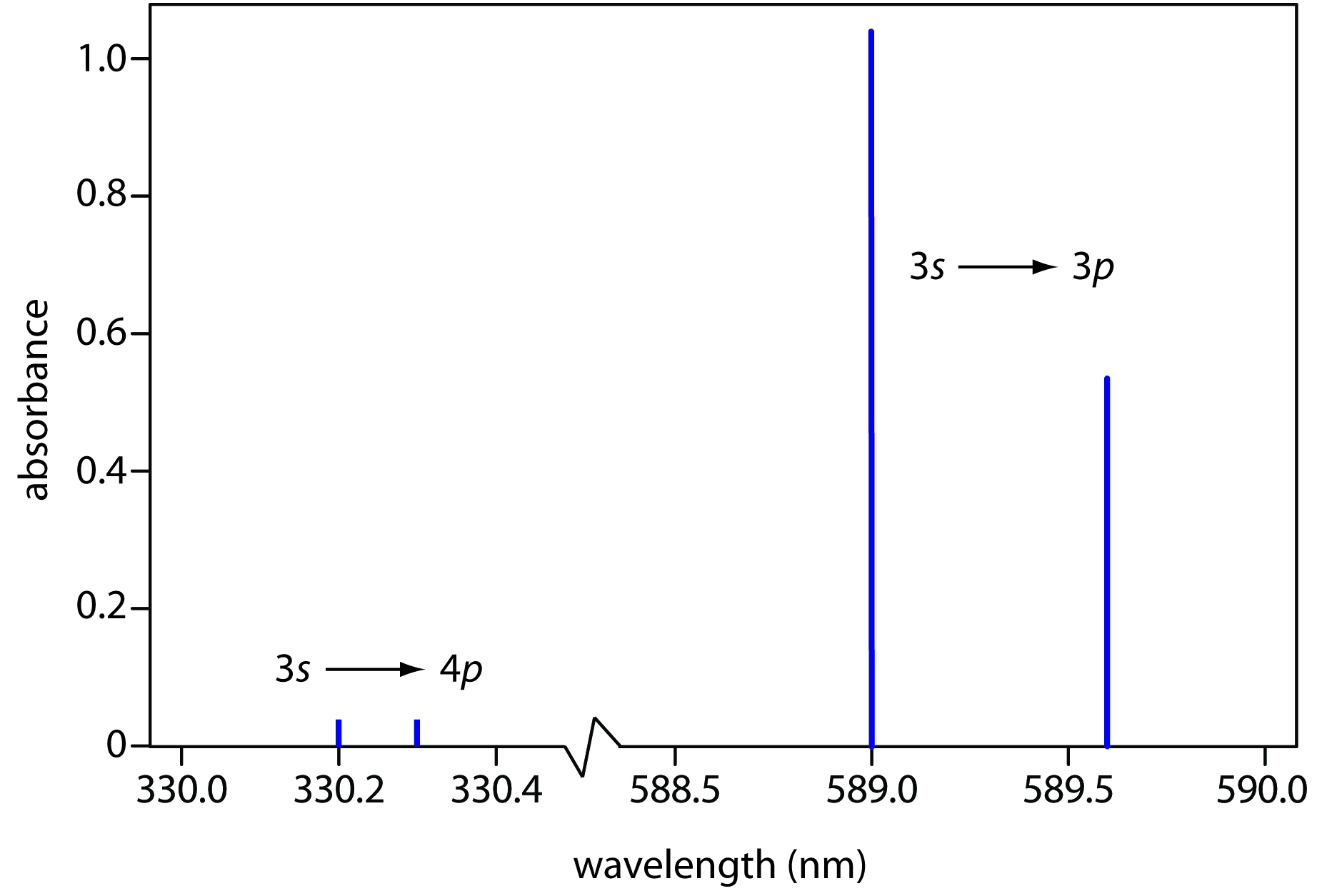
Why is the line spectrum of each element different from others?
Because the energy absorbed by the electrons of two different elements is different. So naturally the energy released by them which forms line spectra will also be different. Hence line spectrum of each element is unique and does not correspond to any other element’s spectrum.
Why do different elements have different spectra?
Why do different elements have different spectra? Each elements emission spectrum is distinct because each element has a different set of electron energy levels. The emission lines correspond to the differences between various pairs of the many energy levels.
Why do lighter elements show more spectral lines than heavier elements?
It's easier to excite electrons of lighter metals like hydrogen, helium, lithium since they possess a few electrons anyways so they show spectral lines easily than heavier elements.
What is the difference between line spectra and band spectra?
Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from one energy level to another. Band spectra, or molecular spectra, are produced by molecules radiating their rotational or vibrational energies, or both simultaneously.
Why are the spectra of two elements different?
Each element has a different set of allowed orbits, so each element emits or absorbs photons with different energies -- and therefore, different wavelengths.
Are atomic line spectra unique to each element?
Each element produces a unique set of spectral lines. Since no two elements emit the same spectral lines, elements can be identified by their line spectrum.
Why does each element have its own unique atomic line spectrum multiple choice question?
Each element has its own atomic line spectrum, consisting of fine lines of individual wavelengths that are characteristic for the element. This occurs because the atom contains specific levels, and an atom can only absorb or emit radiation that corresponds to the energy between these levels.
Why does each element displays a unique gas phase emission spectrum?
(d) Each element displays a unique gas-phase emission spectrum. Each element has a unique set of quantized energy states for its electrons (because of its unique nuclear charge and unique electron configuration).
Why do dark lines appear in absorption spectra?
The lines in the absorption spectrum are dark because that element uses that particular wavelength of light to be absorbed in order to jump to higher shells in its atom.
Why is the emission spectrum of each element different?
Each elements emission spectrum is distinct because each element has a different set of electron energy levels. The emission lines correspond to the differences between various pairs of the many energy levels. The lines (photons) are emitted as electrons fall from higher energy orbitals to lower energies.
Why do elements have different colors?
Because each element has a different set of emission colors from the emission spectrum. They represent the wavelengths of light that is absorbed by the spectrum They represent the energy that the atom gives off when their electrons are in place. They also represent frequency and energy.
What is the emission spectra of a gas?
An emission spectra occurs when the atoms and molecules in a hot gas emit extra light at certain wavelengths, causing bright lines to appear in a spectra. As with absorption spectra, the pattern of these lines are unique for each element.
Why is the emission spectrum of each element different?
Each elements emission spectrum is distinct because each element has a different set of electron energy levels. The emission lines correspond to the differences between various pairs of the many energy levels. The lines (photons) are emitted as electrons fall from higher energy orbitals to lower energies.
Why do elements have different colors?
Because each element has a different set of emission colors from the emission spectrum. They represent the wavelengths of light that is absorbed by the spectrum They represent the energy that the atom gives off when their electrons are in place. They also represent frequency and energy.
What is the emission spectra of a gas?
An emission spectra occurs when the atoms and molecules in a hot gas emit extra light at certain wavelengths, causing bright lines to appear in a spectra. As with absorption spectra, the pattern of these lines are unique for each element. We can see emission spectra from comets, nebula and certain types of stars.
Which spectroscopy is used to obtain line spectra?
Actually which spectroscopy you are talking it's quite confusing but these line spectra are normally obtained in atomic absorption spectroscopy because in atomic absorption spectroscopy electronic transistion take place and it's of discrete levels so here hollow cathode lamp is used which is made up of same material as that of analyte we used so that why every atom give different atomic spectra
Why is the spectrum of an atom unique?
The spectrum of each element is unique because each element has a unique electronic configuration. The frequency of a scattered photon is determined by a change in the quantum energy level of the scattering electron, and the energy levels that are possible are mainly determined by the number of electrons (and protons) bound by the atomic nucleus. Thus, the spectra of an ionized atom differs from that that of the neutral atom. Incidentally, the spectra of an atom in a very strong electric or magnetic field differs from that of an atom outside of the field, again because of the permitted changes in quantum energy levels, but the deviation in frequencies from the normal spectrum are relatively small..
What happens when you irradiate an element?
When you irradiate atom of an element with some energy waves , the atom abdorbs that energy to excite its electron to higher energy levels and it will then come back to its ground energy level radiationg some amount of energy which you take it as its Emission spectrum/spectra.
Why is the spectrum of each element unique?
The spectrum of each element is unique because each element has a unique electronic configuration. The frequency of a scattered photon is determined by a change in the quantum energy level of the scattering electron, and the energy levels that are possible are mainly determined by the number of electrons (and protons) bound by the atomic nucleus. Thus, the spectra of an ionized atom differs from that that of the neutral atom. Incidentally, the spectra of an atom in a very strong electric or magnetic field differs from that of an atom outside of the field, again because of the permitted changes
What is discrete spectra?
Discrete spectra are associated with transitions between energy states in which all electrons are bound. The spectrum is continuous when electrons are unbound. According to modern physics, change in the state of an electron results in radiation either bring absorbed or emitted. Acceleration leads to change in electron state. Hence, if the electron is trapped always to move in a circle, centrifugal Force counts as an acceleration. And radiation is emitted continuously at a rate determined by the radius of the circle and the energy that must be extracted from the Applied fields, inter Alia. This is called synchrotron radiation. Back to bound states with discrete spectra. The energy difference between bound states that are allowed. Discretely from quantum mechanics, depend on the mass and structure of the nucleus, and the potential energy strength between the nucleus and the electrons. Only discrete transitions are allow, a fundamental aspect of quantum mechanics. The radiation emitted when transitioning from one state to another is also at a discrete frequency, apart from Doppler Shifts, and similar perturbations that induce apparent continuous line shapes. The frequencies are characteristic of the properties of the nucleus and the numbers of electrons distributed among the various “shells.” According to quantum mechanics, there is a maximum number of electrons allowed in each bound shell. This is why different elements have different discrete emission frequencies. The energy state, essentially a function of temperature, determines which states are excited and which frequencies are admitted. Quantum mechanics works.
Why are orbital energies different in two different elements?
Two different elements have different nuclei. Hence different orbital energies. So if an electron from the 2 shell jumps to the first shell in both the elements. .the energy gap isnt gonna be the same simply cuz the energy of the lower orbitals are different.
How to find out what kind of atoms are in a star?
The estimation about the kind of atoms present in a star is done through studying the type of light received from it. The spectrum of the light falling on our telescopes and detectors is observed and noted. It is generally seen that some colours of specific frequencies are missing from the spectrum of light. It is due to the fact that these colours were absorbed by the constituent atoms, which make up the star. The remaining frequencies are reflected back to the earth. Thus, we figure out the constituent elements of the star by using spectroscopic methods i,e by observing the light received.
Why do atoms have a line spectrum?
When atoms are excited they emit light of certain wavelengths which correspond to different colors. The emitted light can be observed as a series of colored lines with dark spaces in between; this series of colored lines is called a line or atomic spectra. Each element produces a unique set of spectral lines.
Why do atoms give line spectra while the molecules give band spectra?
Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from one energy level to another. Band spectra, or molecular spectra, are produced by molecules radiating their rotational or vibrational energies, or both simultaneously.
Why are line emission spectra important?
Each element has a different atomic spectrum. The production of line spectra by the atoms of an element indicate that an atom can radiate only a certain amount of energy. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table.
Why do atoms emit line spectra and not continuous spectra?
Quick answer: Atomic spectra are continuous because the energy levels of electrons in atoms are quantized. The electrons in an atom can have only certain energy levels. Each packet of energy corresponds to a line in the atomic spectrum. There is nothing between each line, so the spectrum is discontinuous.
Can an atom collapse?
As a result, each electron in a stable atom remains in its spread-out wavefunction shape. Each electron continues to flow in, out, and around the nucleus without finding anything in the nucleus to interact with that would collapse it down inside the nucleus.
Which property can we identify using emission spectra?
In emission spectra, bright lines will show up corresponding to the difference between energy levels of the elements, where in an absorption spectrum, the lines will be dark. By looking at the pattern of lines, scientists can figure out the energy levels of the elements in the sample.
Do molecules give line spectra?
One might expect the spectra of molecules to be like the atomic line spectra shown in Figure 21.6. 1, but in fact molecular spectra are very different. Instead of the few discrete lines typical of atoms, we now have a broad, apparently continuous, absorption band. This is typical of molecules.