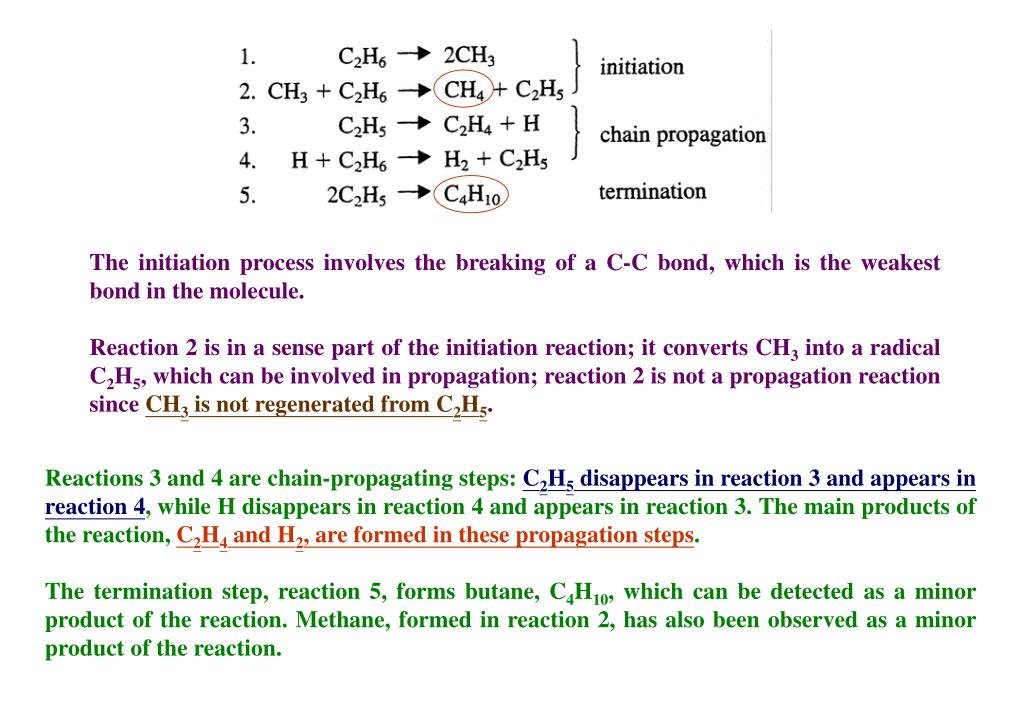
What are some examples of weak chemical bonds?
3 rows · Jun 01, 2020 · They are (strongest to weakest) hydrogen bonding, dipole-dipole and Van der Waals' forces. ...
Which is the strongest chemical bond and why?
Jun 08, 2021 · There are three types of bonds that are important to biology: ionic (second strongest), covalent (strongest), and hydrogen ( weakest ). Which bond is the weakest bond? The weakest of the intramolecular bonds or chemical bonds is the ionic bond then polar covalent bond and the strongest is the non-polar covalent bond .
What are the bonds listed strongest to weakest?
Mar 17, 2021 · The weakest bonds are hydrogen bonds. This means that they can easy be broken down. Covalent bonds, metallic bonds, and electrovalent (or ionic) bonds are much stronger bonds. What is the weakest bond in chemistry? The bond which is the weakest bond in chemistry is wander wal forces or London dispersion forces……..
What are the 5 types of chemical bonds?
Nov 25, 2020 · Chemical bonds are formed when the electrons in an atom interact with the electrons in another atom. Strong bonds form when two atoms share electrons. The weakest of the intramolecular bonds or chemical bonds is the ionic bond then polar covalent bond and the strongest is the non-polar covalent bond.
What are weak bonds called?
Definition. Weak bonds are those forces of attraction that, in biological situations, do not take a large amount of energy to break. … In biological terms, ionic bonds, hydrogen bonds, and van der Waals interactions are considered weak bonds .
What are the 4 types of bonds?
There are four types of bonds or interactions: ionic, covalent, hydrogen bonds, and van der Waals interactions. Ionic and covalent bonds are strong interactions that require a larger energy input to break apart.
What is the weakest bond in biology?
Thus, we will think of these bonds in the following order (strongest to weakest ): Covalent, Ionic, Hydrogen, and van der Waals. Also note that in Chemistry, the weakest bonds are more commonly referred to as “dispersion forces.”
Is covalent bond a weak bond?
Covalent and ionic bonds are both typically considered strong bonds. However, other kinds of more temporary bonds can also form between atoms or molecules. Two types of weak bonds often seen in biology are hydrogen bonds and London dispersion forces.
What type of chemical bond is considered weak?
The ionic bond is generally the weakest of the true chemical bonds that bind atoms to atoms .
Which bond is stronger ionic or covalent?
Ionic bond is much stronger than covalent bond because it involves complete transfer of electrons because of which there is formation of cation and anion and there exist huge electrostatic forces of attraction. They also have high melting and boiling point which proves that the ionic bond is very strong.
Why ionic bonding is stronger than covalent?
They tend to be stronger than covalent bonds due to the coulombic attraction between ions of opposite charges. To maximize the attraction between those ions, ionic compounds form crystal lattices of alternating cations and anions.
Which bond is the weakest bond of the following?
The Hydrogen bonds are the weakest as they aren’t really bonds but just forces of attraction to the dipoles. On a hydrogen atom which are permanent and bonded to two atoms which are highly electronegative in nature.
What is the strongest to weakest bond?
Complete answer: The order from strongest to weakest bonds is: Covalent bond > ionic bond > hydrogen bond >Van der Waals forces.
What are the two weakest bonds?
Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist.
Are covalent bonds the weakest?
This means Ionic bonds tend to dissociate in water. Thus, we will think of these bonds in the following order (strongest to weakest): Covalent, Ionic, Hydrogen, and van der Waals. Also note that in Chemistry, the weakest bonds are more commonly referred to as “dispersion forces.”.
Which is the most strongest bond?
In chemistry, covalent bond is the strongest bond. In such bonding, each of two atoms shares electrons that binds them together. For example, water molecules are bonded together where both hydrogen atoms and oxygen atoms share electrons to form a covalent bond.
Which combination will give the strongest ionic bond?
Answer: The combination of Mg 2+ and O 2– has the strongest ionic bond because it has high lattice energy among all the given options.
What is the easiest chemical bond to break?
A hydrogen bond is the chemical bond that is the easiest chemical bond to break.
What is chemical bond?
Explanation: A Chemical bond is technically a bond between two atoms that results in the formation of a molecule , unit formula or polyatomic ion.
Is hydrogen bond a chemical bond?
The " Hydrogen Bond" is not actually a chemical but an intermolecular force or attraction. Other intermolecular forces are the Van der Walls interactions and the dipole dipole attractions. The ionic bond is generally the weakest of the true chemical bonds that bind atoms to atoms.
What is the order of the strength of bonds from weakest to strongest?
This means Ionic bonds tend to dissociate in water. Thus, we will think of these bonds in the following order (strongest to weakest): Covalent, Ionic, Hydrogen, and van der Waals. Also note that in Chemistry, the weakest bonds are more commonly referred to as “dispersion forces.”
Which bond is the strongest and weakest?
Of the 4 different types of chemical bonds, covalent bonds are known to be the strongest and the bonds formed via Van der Waals forces are known to be the weakest. The ranking is: Covalent bond > ionic bond > hydrogen bond > Van der Waals forces. Was this answer helpful?
What is the strongest bond ionic or covalent?
Ionic bonds are stronger than covalent bonds, because there is a stronger attraction between ions that have opposite charges, which is why it takes a lot of energy to separate them. Covalent bonds are bonds that involve the sharing of electron pairs between atoms.
Which ionic bond is the strongest?
Ionic Bonds The strength of the ionic bond is directly dependent upon the quantity of the charges and inversely dependent on the distance between the charged particles. A cation with a 2+ charge will make a stronger ionic bond than a cation with a 1+ charge.
Which chemical bond is strongest?
Covalent bond is the strongest bond. Answer: There are a variety of ways atoms bond to one another. Some bonds are weaker, and some are stronger.
What are the two types of chemical bonds?
Chemical bonds include covalent, polar covalent, and ionic bonds. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds. Atoms with large differences in electronegativity transfer electrons to form ions.
What are the three major types of chemical bonds?
There are three primary types of bonding: ionic, covalent, and metallic.