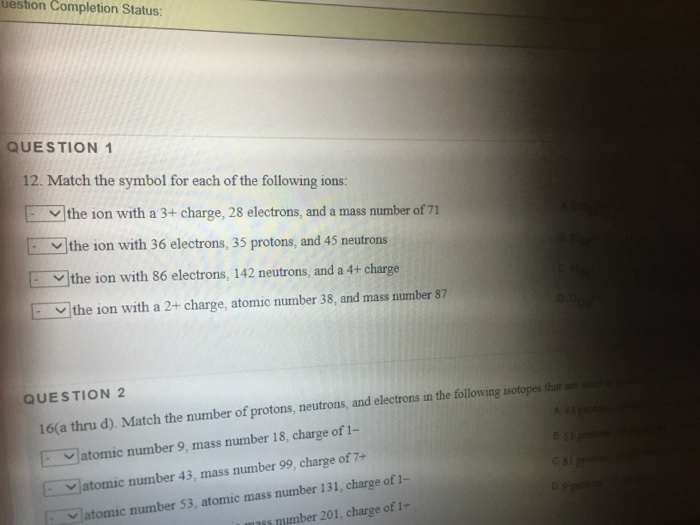
What is the symbol for the ion with 36 electrons?
What is the symbol for the ion with 36 electrons 35 protons and 45 neutrons? Name Gallium Symbol Ga Atomic Number 31 Atomic Mass 69.723 atomic mass units Number of Protons 31
Which of the following element has 90 protons?
The element with 90 protons is thorium (Th). The symbol is ^232Th^4+. (d) Strontium has atomic number 38 and the symbol is ^87Sr^2+.
How many protons and electrons are in a Ga3+ ion?
(a) Gallium has an atomic number of 31 and would form a 3+ ion with 28 electrons. The complete symbol is ^71Ga^3+. (b) The element with 35 protons is bromine. Its 36 electrons are consistent with a single negative charge. The complete symbol is ^80Br^-.
How many protons are in the ion ^71Ga^3+?
(a) Gallium has an atomic number of 31 and would form a 3+ ion with 28 electrons. The complete symbol is ^71Ga^3+. (b) The element with 35 protons is bromine.
What atom is represented by 35 protons 45 neutrons and 35 electrons?
BromineThe mass number = protons + neutrons. Bromine has a mass number of 80 and 35 protons so 80-35 = 45 neutrons. b) How many electrons does the neutral atom of bromine have? The neutral atom of bromine has 35 electrons because the number of electrons equals the number of protons.
What is the symbol of the ion with 35 protons and 36 electrons and 37 neutrons?
Bromine is a chemical element with symbol Br and atomic number 35.Dec 19, 2021
What is the symbol and charge for the ion that has 35 protons and 36 electrons?
For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. This results in an anion with 35 protons, 36 electrons, and a 1− charge. It has the same number of electrons as atoms of the next noble gas, krypton, and is symbolized Br−.Jun 15, 2020
What is the symbol for the ion containing 34 protons 46 neutrons and 36 electrons?
We represent this selenide ion as Se2− .Dec 19, 2015
What is the nuclear symbol for ion containing 34 protons 45 neutrons and 36 electrons?
We represent this selenide ion as Se2− .Aug 12, 2020
What is the charge of an ion that has 35 protons 38 neutrons and 36 electrons?
The net charge on the ion is the difference between the two: Ion charge = +35 - 36 = -1.
Which isotope has 35 protons 35 electrons and 46 neutrons?
For this bromine isotope, the mass number is 35 + 46 or 81.Apr 24, 2017
What is an ionic symbol?
Conventions for Writing Ions When writing the symbol for an ion, the one- or two-letter element symbol is written first, followed by a superscript. The superscript has the number of charges on the ion followed by a + (for positive ions or cations) or - (for negative ions or anions).Nov 19, 2019
What element has 36 electrons when it forms an anion with a 1 − charge the elemental symbol of this element is?
bromineFor example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. This results in an anion with 35 protons, 36 electrons, and a 1− charge. It has the same number of electrons as atoms of the next noble gas, krypton, and is symbolized Br−.
What has 34 protons and 45 neutrons?
Selenium has 34 protons and 45 neutrons in its nucleus giving it an atomic number of 34 and a atomic mass of 79. Selenium is in Period 4 of the Periodic Table because it has 4 electron shells.
What is the ionic charge of an ion with 38 protons and 36 electrons?
The atomic number (38) is the number of protons. Since the charge on the ion is +2, we know there are two more protons than electrons. Hence 36 electrons.Dec 14, 2017
What isotope has 36 electrons in an atom?
Which isotope has 36 electrons in an atom quizlet? Krypton is neutral with a mass number of 84, 36 protons, 48 neutrons, 36 electrons, and 0 charge.Jan 4, 2022
What is the difference between protons and electrons?
Although charges are the same size, the signs of the charges are opposite. Protons are much more massive than electrons. Protons are located within an atom's nucleus , whereas electrons are located outside the nucleus.
Which is more massive, electrons or protons?
Protons are much more massive than electrons. Protons are located within an atom's nucleus, whereas electrons are located outside the nucleus. In the following symbols, both atomic number and mass number are given if available from the problem. (a) Gallium has an atomic number of 31 and would form a 3+ ion with 28 electrons.
What is the symbol for oxygen?
Explain why the symbol for an atom of the element oxygen and the formula for a molecule of oxygen differ. The symbol for the element oxygen, O , represents both the element and one atom of oxygen.
How many atoms are in O2?
A molecule of oxygen, O2, contains two oxygen atoms; the subscript 2 in the formula must be used to distinguish the diatomic molecule from two single oxygen atoms. Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl4, PCl3, CaCl2, CsCl, CuCl2, and CrCl3.
Is chloride ionic or covalent?
Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl4, PCl3, CaCl2, CsCl, CuCl2, and CrCl3. Covalent compounds are usually formed by a combination of nonmetals. Ionic compounds are usually formed when a metal is combined with one or more nonmetals.