What is the Lewis dot
Lewis structure
Lewis structures (also known as Lewis dot diagrams, electron dot diagrams, Lewis dot formulas, Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
How to Type A Lewis dot structure?
- Arrange the atoms to show specific connections. ...
- Determine the total number of valence electrons in the molecule or ion. ...
- Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. ...
- Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). ...
How do you write a Lewis dot diagram?
aim: how to write lewis dot structures (electron dot structures) do now: 1. read both sides of the handout. 2. write the electron configuration (orbital notation) of phosphorus atom, and phosphorus ion. 3. draw the lewis dot structure for the atom and the ion.
What is the Lewis dot diagram purpose?
- Electron dot diagram is known Lewis dot structure.
- It is helpful in understanding structure of compound and electron in 2D about there electrons.
- I hope it may help u.
What does a Lewis dot structure do?
Lewis Structures are important to learn because they help us predict:
- the shape of a molecule.
- how the molecule might react with other molecules.
- the physical properties of the molecule (like boiling point, surface tension, etc.).
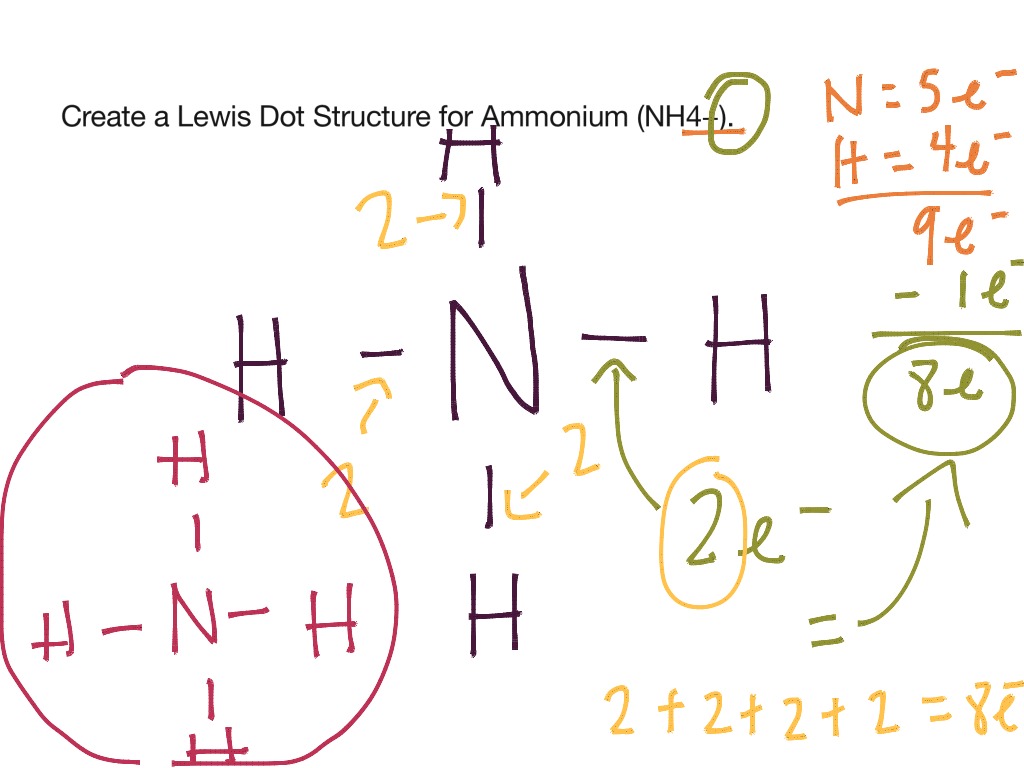
What is the Lewis structure of ammonium?
0:442:34NH4+ Lewis Structure - Ammonium Ion - YouTubeYouTubeStart of suggested clipEnd of suggested clipNo dots or no lone pairs. So five minus four is plus one thus the positive charge that we see in theMoreNo dots or no lone pairs. So five minus four is plus one thus the positive charge that we see in the ammonium ion is attributable to the formal charge in the nitrogen atom.
Which of the following is the correct Lewis dot structure for ammonia?
0:101:31How to Draw the Dot Structure for NH3 (Ammonia) - YouTubeYouTubeStart of suggested clipEnd of suggested clipEach one of those has two valence electrons. So we're good that's the lewis structure for nh3.MoreEach one of those has two valence electrons. So we're good that's the lewis structure for nh3.
What is the Lewis dot structure for h2o?
0:001:21How to Draw the Lewis Structure for Water - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo 1 times 2 is 2 plus 6 2 plus 6 equals 8 we have a total of 8 valence electrons we'll put theMoreSo 1 times 2 is 2 plus 6 2 plus 6 equals 8 we have a total of 8 valence electrons we'll put the oxygen in the center and hydrogen's always go on the outside.
What is the bonding formation of ammonia?
covalent bondsExplain the formation of ammonia molecule. Ammonia is formed from 3 atoms of Hydrogen and 1 atom of Nitrogen. Hence, three hydrogen atoms each share their 1 electron with nitrogen to form three covalent bonds and make an ammonia molecule (NH3) ammonia molecule.
Does ammonia have a charge?
Ammonia has zero charge because it is a neutral molecule that consists of a nitrogen atom bonded to three hydrogen atoms by covalent bonds. There i...
Does NH4 have a charge?
NH4 has a positive charge on its central atom ( i.e., the nitrogen) as the unpaired electrons on nitrogen are used to form a bond with the proton....
What is the difference between ammonium and ammonia?
Ammonia is a neutral species that comprises a nitrogen atom attached to three hydrogen atoms while ammonium is a positively charged species that co...
Is ammonium an acid?
Ammonium can generate hydrogen ions on dissociation and hence it is considered an acid. Ammonium is the conjugate acid of the weak base ammonia.
What is ammonium cation charge?
The ammonium cation bears a positive charge as the lone pair of electrons on nitrogen atom (in ammonia) is lost while forming the bond with the pro...
Why does ammonium have a charge of 1?
Ammonium has a charge of 1 as the lone pair of electrons on the nitrogen atom is used to form a bond with hydrogen. Since the electrons on the nitr...
What are Lewis structures?
Lewis structures are the basic representation of the distribution of valence electrons, lone pairs, and charges on atoms, molecules, and ions. They also depict the bonding between the atoms in a molecule. These representations/structures are also known as Lewis dot structures as dots are used to signify the electrons, and a pair of dots or a line is used to indicate a bond.
What is the representation of the constituent atoms of a compound called?
The representation indicating the constituent atoms (using symbols) of a compound, with their respective proportions, is called a chemical formula. The proportion of each element is indicated as its subscript, and overall charge is shown in superscript.
What is Lewis structure?
A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots. A bond between two electrons is represented by a line marked by a dot at both ends, involving the participating electrons. The end goal is to identify a configuration with ...
What are the properties of an atom identified through molecular geometry?
The properties of an atom identified through molecular geometry help in understanding the behavior, utility, and reactivity of the element. Depending upon their geometry, various molecular structures can be classified into linear, angular, trigonal planar, octahedral, trigonal pyramidal, among others. One can draw the 3-dimensional structure of an ...
How are hybrid orbitals formed?
These hybrid orbitals, formed by the hybridization of an atom , are helpful in the explanation and understanding of an atom’s molecular geometry, its atomic bond properties, and the position in the atomic space. In most common scenarios, atomic orbitals with similar energy combine to form hybrid orbitals.
What is the hybridization of NH4?
The Hybridization of NH4. The concept of Hybridization decrees that atomic orbits fuse with one another to form new degenerated hybrid orbitals, which influence bonding properties and molecular geometry of the atoms of an element.
What is the chemical formula for NH3?
NH3 is the chemical formula of Ammonia. A positively charged polyatomic ion of Ammonium or NH4+ comes into existence when an Ammonia atom goes through the process of protonation, that is, it loses one of its electrons and becomes positively charged.
How many electrons does NH4+ have?
As mentioned earlier, NH4+ is made up of Nitrogen and Hydrogen. If we look towards the periodic table, we will find Hydrogen in group 1. This means that Hydrogen has 1 electron. NH4+ has 4 hydrogen atoms, therefore, there are 4 hydrogen electrons.
How many valence shell electrons does NH4+ have?
Nitrogen, having 5 valence shell electrons, along with 4 from Hydrogen, should have had 9 electrons. But the + sign decrees that NH4+ has 8 valence shell electrons, due to the positive ion.