How do you write a balanced chemical equation?
Jun 09, 2015 · As a result, the chemical equation that shows the chemical reaction needs to be balanced. A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to...
How can you tell if a chemical equation is balanced?
Apr 12, 2020 · A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. Also Known As: Balancing the equation, balancing the reaction, conservation of charge and mass.
What does it mean when a chemical equation is balanced?
Dec 10, 2008 · A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. In other words, the mass and the charge are balanced on both sides of the reaction. Also Known As: Balancing the equation, balancing the reaction, conservation of …
What must happen for a chemical equation to be balanced?
Jun 02, 2020 · The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation. The Law of conservation of mass governs the balancing of a chemical equation. According to this law, mass can neither be created nor be destroyed in a chemical reaction and obeying this law total mass of the …
What is balanced chemical equation short definition?
Updated on November 07, 2019. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. In other words, the mass and the charge are balanced on both sides of the reaction.Nov 7, 2019
What is balanced chemical equation class 10?
Answer: The representation of a chemical reaction in the form of substances is known as a chemical equation. The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation.
What is balanced chemical equation class 11?
Hint: Balanced chemical equations are those in which the number of atoms on the reactant side is equal to the number of atoms on the product side.
Which is a balanced chemical equation Brainly?
Answer. The reaction in which the number of atoms of each element is equal on the reactant side and product side is called balanced chemical reaction.Feb 27, 2020
Why is an equation balanced?
The equation is balanced for charge because both sides of the equation have no ions (net neutral charge). The equation has 2 iron atoms on the reactants side of the equation (left of the arrow) but 1 iron atom on the products side (right of the arrow). Even without counting up the quantities of other atoms, you can tell the equation isn't balanced.
What is balanced equation?
A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. In other words, the mass and the charge are balanced on both sides of the reaction. Also Known As: Balancing the equation, ...
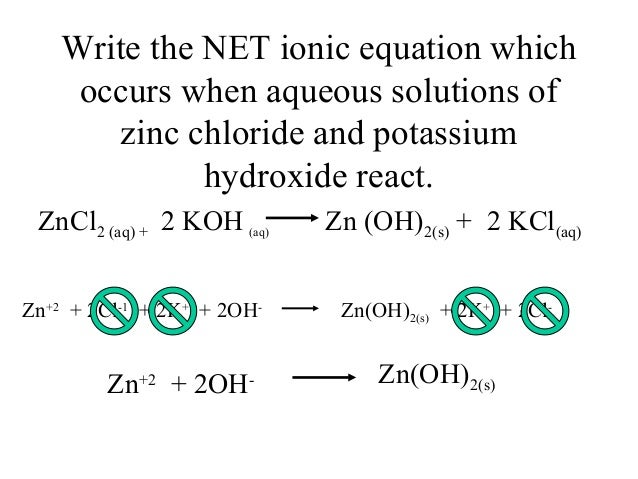
How to check if an ionic equation is balanced?
Check that an ionic equation is balanced for the charge by seeing if all the positive and negative charges cancel each other out on each side of the equation. For example, on the left side of the equation, there are 2 positive charges and 2 negative charges, which means the net charge on the left side is neutral.
What are some examples of unbalanced and balanced equations?
For example, this equation for the reaction between iron oxide and carbon to form iron and carbon dioxide is unbalanced with respect ...
What are the subscripts of an equation?
Changing the subscripts would alter the chemical identity of the compound. Both the left and right sides of the equation have 4 Fe, 6 O, and 3 C atoms.
What does balancing for charge mean?
Balancing for charge means the net charge is zero on both sides of the equation. The state of matter (aq) stands for aqueous, meaning only the ions are shown in the equation and that they are in the water. For example: Ag + (aq) + NO 3- (aq) + Na + (aq) + Cl - (aq) → AgCl (s) + Na + (aq) + NO 3- (aq)
What is a balanced chemical reaction?
A balanced chemical reaction is an equation that has equal numbers of each type of atom on both sides of the arrow. A chemical equation is a written symbolic representation of a chemical reaction. The reactant chemical (s) are given on the left-hand side and the product chemical (s) on the right-hand side. The law of conservation of mass states ...
What is the law of conservation of mass?
The law of conservation of mass states that atoms can neither be created nor destroyed in a chemical reaction, so the number of atoms that are present in the reactants has to balance the number of atoms that are present in the products.