What is the bond order of co+?
But the bond order for CO+ is increased to 3.5. This is because the last electron occupies the antibonding orbital. Therefore the bond order of CO+ is 3.5 Hope this will be helpful. Bbond order of CO+ is 3.5
What is the bond order of carbon and oxygen in carbon dioxide?
In order to achieve a stable configuration, two of oxygen's electrons are shared and form a covalent bond by themselves (a dative bond), allowing the carbon to achieve a stable configuration. Now that both carbon and oxygen have filled shells, the molecule is stable. Since there are three bonds between the two atoms, the bond order is 3.
What is bond order in organic chemistry?
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication of the stability of a bond.
How to calculate bond order of CO32?
How To Calculate Bond Order Of CO32- Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. The Bond Order Formula is [latex]frac {1} {2} [N_ {b}- N_ {a}] latex]
What is the bond order of Cu group?
The Bond Order in CO+ is 3.5 .Sep 27, 2018
What type of bond is CO?
triple covalent bondThe carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. One of the bonds is a coordinate covalent bond, a covalent bond in which one of the atoms contributes both of the electrons in the shared pair.Feb 21, 2022
How do you find the bond order of CO+?
Measured CO bond length is 1.128 Å, & bond length of CO+ is 1.115 Å.In the case of CO, the 2s atomic orbital on Oxygen is much lower in energy than the 2s atomic orbital in carbon.Bond Order of CO+ = (8-1)/2 = 3.5.
How many bonds are there in CO?
Hint: There are two covalent bonds and one coordinate bond present in Carbon monoxide molecules.
What is the bond energy of C O?
Common Bond Energies (DBondD (kJ/mol)r (pm)C≡N887116C-P264184C-O358143C=O79912017 more rows
Why is bond order 3.5 in CO+?
The BO in CO+ is 3.5 because the order of the orbitals is not the same as it would be for a homonuclear diatomic molecule such as O2where both atoms are the same. Total number of electrons = 6 + 8 = 14. The bond order of CO is 3.Sep 8, 2019
What is the bond order of CO and NO+?
So, from the above discussion now we know that the bond order of carbon monoxide and nitric oxide molecules is 3 and 2.5 respectively.
What is the bond length of CO?
∼1.43 ÅC-O bonds in ethers are generally ∼1.43 Å in length, but oxatriquinane has been found to have C-O bond lengths of 1.54 Å.
What is the bond order of CO+?
The bond order of CO+ molecule is predicted to be 2.5 according to Harry B. Gray's book Electrons and Chemical Bonding. You can refer to the book for molecular diagrams of other diatomic and triatomic molecules and their bonds.
Why is the bond order for CO+ increased to 3.5?
But the bond order for CO+ is increased to 3.5. This is because the last electron occupies the antibonding orbital.
How many electrons does CO+ have?
In CO the p orbitals are fully filled that makes it stable while in case of CO+ it has 5 electrons.
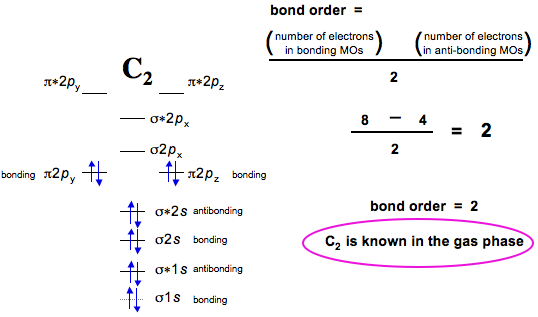
How many electrons does a species have in a bonding MO?
As per MO description, the species has 9 electrons in the bonding MOs, and 4 in the antibonding MOs.
How to find the total number of valence electrons in an octet state?
Find total number of valence electrons for Octet state (simply multiply 8 to total number of atoms in question). For the given question it's 8*2=16.
How many electrons are in a co+9 molecule?
In co+,9 electrons are present inbonding molecule and 4 in anti-bonding molecule…
Is the order of energy of the orbitals the same as it would be for a homonuclear?
The order of energy of the orbitals is “not” the same as it would be for a homonuclear diatomic molecule such as O2 where both atoms are the same.
How many electrons can be filled in carbon monoxide?
While filling in the final complex’ electrons, we start from the bottom; first the twelve ligand electrons that represent the carbon monoxide lone pairs. Once we are done with this, the next orbitals happen to be t 2 g — the bonding ones which have more ligand contribution. These can not only be seen as bonding with respect to the M ← L bond but also antibonding with respect to the C = O bond. The maximum we can fill in here is 6 electrons. These six electrons reduce the C ≡ O bond order as can be seen in the formula:
What is the meaning of "back up"?
Making statements based on opinion; back them up with references or personal experience.
Why is factor 6 added to the denominator?
The additional factor 6 in the denominator is because I am analysing six C ≡ O bonds at the same time.
Which side of a carbonyl complex is higher in energy?
In a metal carbonyl complex, the left-hand side is usually higher in energy due to the metal’s lower oxidation state. Thus, the lower an orbital in the centre of the scheme is, the more ligand-centred it is.
Can octahedra be done with any geometry?
The discussion I did with an octahedral complex can be done with any coordination geometry; octahedra are just a very typical case.
What is bond order?
Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond.
How many bond groups are there between atoms?
The number of bond groups between individual atoms is 3.