| Bent molecular geometry | |
|---|---|
| Examples | H2O, SO2 |
| Point group | C2v |
| Coordination number | 2 |
| Bond angle(s) | 90°<θ<120° |
How do I determine the bond angle in a molecule?
Vocabulary
- Electron Geometry: Describes the arrangement of bonds and lone pairs around a central atom.
- Molecular Geometry: Describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons.
- Hybridization: Orbitals are combined in order to spread out electrons.
- Bond angles: The angle between adjacent bonds of an atom.
What determines bond angle in a molecule?
Bond Angle. Bond angle is the angle between two bonds that are connected to the same atom. A molecule with only two atoms doesn't have a bond angle; there needs to be at least three atoms before ...
How would I know the bond angles in a molecule?
- Get the group number of the primary element
- If its a negative charge, add that to the group number from 1) or subtract the positive charge
- Add the bonded pairs
- Divide by 2 to get the bonded pairs
- Now subtract the bonded pairs by the number of bonds to find the loan pairs.
How to determine the bond shape of a molecule?
geometry, or three-dimensional shape of a molecule or polyatomic ion, can be determined using valence-shell electron-pair repulsion (abbreviated VSEPR and pronounced “VES-per”) theory, in which the basic principle is valence electrons around a central atom stay as far apart as possible to
What is the bond angle of bent?
approximately 109.5o.NOTES: This molecule is made up of 4 equally spaced sp3 hybrid orbitals forming bond angles of approximately 109.5o. The shape of the orbitals is tetrahedral. Two of the orbitals contain lone pairs of electrons.21-Aug-2020
What is the bond angle in a bent molecule such as water?
The actual bond angle in the water molecule is 104.5°. The opening of the angle to a value greater than the predicted one of 90° can be accounted for in terms of a lessening of the repulsion between the hydrogen nuclei.
Why is the molecular shape bent?
In order to minimise electron-electron repulsions, these pairs adopt a tetrahedral arrangement around the oxygen. It does not matter which two are lone pairs and which two are connected to hydrogen atoms; the resulting shape is always bent.14-Apr-2020
Is bent shape planar?
The answer actually is that the shape is angular, which also means bent. However, it goes further to say that it is in a trigonal planar arrangement. I think what it's trying to explain is that its basic geometry is trigonal planar because the central atom consists of 3 regions of electron density.14-Nov-2018
How do you find bond angles?
1:205:27Predicting Bond Angles - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo whenever you see a molecule that looks like this. It has a 2d structure and if you take a fullMoreSo whenever you see a molecule that looks like this. It has a 2d structure and if you take a full circle which has an angle of 360 divided by 3 you're going to get 120.
How do you find the bond angle of water?
0:182:35Bond Angles for H2O (Ideal and Actual) - YouTubeYouTubeStart of suggested clipEnd of suggested clipDown on the molecule. We add our second lone pair. And now we end up with this shape for the waterMoreDown on the molecule. We add our second lone pair. And now we end up with this shape for the water molecule with this bond angle here and this is an ideal bond angle.
What is the bond angle for tetrahedral?
109.5 degreesThe proof that a bond angle in a tetrahedral molecule is 109.5 degrees is more complex than it first appears.01-Oct-1988
What is meant by bent molecule?
The term bent molecule refers to the physical arrangement of atoms within a molecule. ... A lone pair is where the electrons are not shared with any other atom. That's important for understanding bent molecule structure because a molecule always has a central atom, with 2 bonded atoms, and 1 to 2 lone pairs of electrons.
How do you know a molecule is bent?
If all electrons pairs are acting as bonding electrons, leaving no lone pair on the central atom (AX2), then the molecule will be linear (eg; CO2). If, however, there is one or two lone pairs (E) on the central atom, then the molecule will be bent (AX2E eg; SO2, AX2E2 eg; H2O).
Which molecule is bent with an approximate angle of 120 degrees?
The bond angle in an AX2E molecule is approximately 120 °. This is an AX5 molecule, and its geometry is trigonal pyramidal. The equatorial bond angles in a trigonal bipyramidal molecule are all 120 °.09-Feb-2016
How do you draw a bent molecular geometry?
3:3512:15Easy molecular geometry - YouTubeYouTubeStart of suggested clipEnd of suggested clipPoint five degree angle between these four connected molecules like the two hydrogen's and the twoMorePoint five degree angle between these four connected molecules like the two hydrogen's and the two electrons. So in this case we call it a bent molecule now let's give a couple other examples.
Are bent molecules polar?
Water is a bent molecule because of the two lone pairs on the central oxygen atom. The individual dipoles point from the H atoms toward the O atom. Because of the shape, the dipoles do not cancel each other out and the water molecule is polar.
How do you know if a molecule is bent or linear?
1st remember the names: The names can be determined by the shape and angle of the molecule. Linear = is just a line of atoms with a 180° angle. Not...
What causes a molecule to be bent?
The reason water has a bent shape is that the two lone pair of electrons are on the same side of the molecule. ... This repulsion of the lone pairs...
What is the shape of a bent molecule?
In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Cer...
How do you determine the shape of a molecule?
The shape of a molecule is determined by the location of the nuclei and its electrons. The electrons and the nuclei settle into positions that mini...
What is a bent structure?
A bent in American English is a transverse rigid frame (or similar structures such as three-hinged arches). ... Rather, bents are simply cross-sect...
How many electrons does sulfur have?
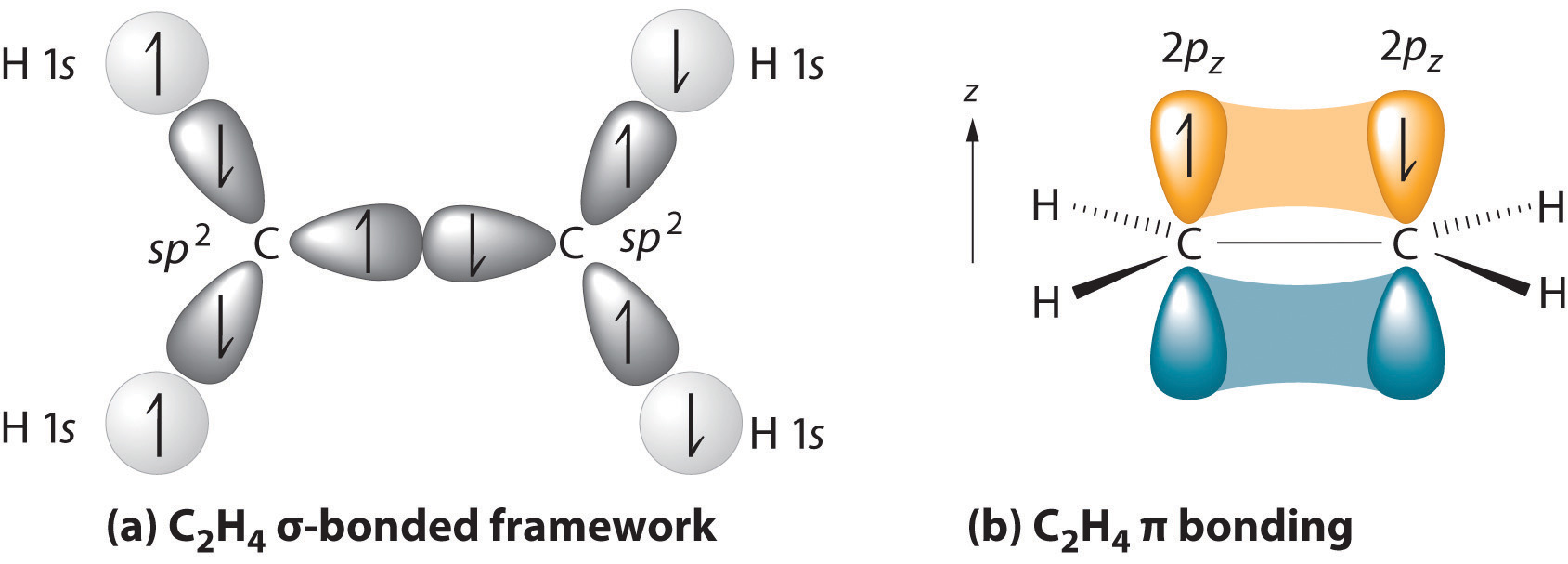
In sulfur dioxide, the sulfur atom is bonded to two oxygen atoms and has one unshared pair of electrons. In formaldehyde and ethylene, each carbon atom has two single bonds to hydrogen, a double bond to another atom, and no unshared pair. The sulfur atom in sulfur dioxide and the carbon atom in ethylene and formaldehyde is surrounded by three ...
What is the Lewis structure of CH4?
Let us predict the shape of methane, CH4. The Lewis structure of methane shows a central atom surrounded by four separate regions of high electron density. Each region consists of a pair of electrons bonding the carbon atom to a hydrogen atom.
What is the shape of sulfur dioxide?
Just as the unshared pair of electrons in sulfur dioxide contribute to the geometry of the molecule but are not included in the description of its geometry, the unshared pair of electrons in ammonia gives it a tetrahedral shape but its geometry is based only on the arrangement of atoms, which is pyramidal.
What is the bond between oxygen and hydrogen?
Two of these regions contain a pair of electrons forming a covalent bond between oxygen and hydrogen; the other two regions contain an unshared electron pair.
How many electron clouds are there in sulfur dioxide?
The whole molecule is planar, and its shape resembles two triangles joined point to point. In sulfur dioxide, there are three electron clouds around the sulfur. Only two of these connect two atoms. In the molecule, the oxygen-sulfur-oxygen atoms make a 120° angle. The molecule is bent.
Which molecule has a central atom that is bonded to four other atoms?
A molecule whose central atom is bonded to four other atoms is tetrahedral. One in which the central atom has one unshared pair of electrons and bonds to three other atoms will be pyramidal, and one in which the central atom has two unshared pairs of electrons and bonds to two other atoms will be bent.
Which atom is surrounded by three clouds of high electron density?
The sulfur atom in sulfur dioxide and the carbon atom in ethylene and formaldehyde is surrounded by three clouds of high electron density. For these clouds to be as far as possible from one another, they will form a plane containing the central atom and will emanate from the central atom at angles of 120° to each other.
Core Concepts
In this tutorial, you will learn how to identify the molecular geometry and bond angles of a molecule. You will learn about the more common molecular geometries: tetrahedral, linear, bent, trigonal pyramidal, and trigonal planar – along with their bond angles.
Vocabulary
Electron Geometry: Describes the arrangement of bonds and lone pairs around a central atom.
What is molecular geometry?
Molecular geometry describes the three-dimensional structure of a molecule. Chemists are able to predict the arrangement of atoms and chemical bonds using the valence-shell electron-pair repulsion theory or VSEPR. This theory revolves around the idea that electrons repel each other and therefore will bond accordingly.
Practice Example
The answer is the molecular geometry of water would be bent. Notice there are 4 attachments, or, electron groups surrounding oxygen. This would make the electron geometry tetrahedral. However, this is not the molecular geometry. Two of these attachments are bonds and the other two are lone pairs.
How many orbitals does SnCl 2 have?
They have three sp 2 orbitals.
What is a molecule with a bent coordination geometry?
Oxygen difluoride, an example of a molecule with the bent coordination geometry. In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Certain atoms, such as oxygen, will almost always set their two (or more) covalent bonds in non-collinear directions due ...
What is bent geometry?
Bent molecular geometry. Oxygen difluoride, an example of a molecule with the bent coordination geometry. In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Certain atoms, such as oxygen, will almost always set their two ...
What are the angles of the sp3?
The most common actual angles are 105°, 107°, and 109°: they vary because of the different properties of the peripheral atoms (X).
Is water a nonlinear molecule?
Certain atoms, such as oxygen, will almost always set their two (or more) covalent bonds in non-collinear directions due to their electron configuration. Water (H 2 O) is an example of a bent molecule, as well as its analogues. The bond angle between the two hydrogen atoms is approximately 104.45°. Nonlinear geometry is commonly observed ...
CORE Concepts
- In this tutorial, you will learn how to identify the molecular geometry and bond angles of a molecule. You will learn about the more common molecular geometries: tetrahedral, linear, bent, trigonal pyramidal, and trigonal planar – along with their bond angles. If you enjoy this tutorial, feel free to check out our other tutorials on bonding listed below.
Covered in Other Articles
Vocabulary
- Electron Geometry: Describes the arrangement of bonds and lone pairs around a central atom.
- Molecular Geometry: Describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons.
- Hybridization:Orbitals are combined in order to spread out electrons.
- Bond angles: The angle between adjacent bonds of an atom.
What Is Molecular Geometry?
- Molecular geometry describes the three-dimensional structure of a molecule. Chemists are able to predict the arrangement of atoms and chemical bonds using the valence-shell electron-pair repulsion theory or VSEPR. This theory revolves around the idea that electronsrepel each other and therefore will bond accordingly.
Practice Example
- What is the molecular geometry and bond angle of water (H2O)?
The answer is the molecular geometry of water would be bent. Notice there are 4 attachments, or, electron groups surrounding oxygen. This would make the electron geometry tetrahedral. However, this is not the molecular geometry. Two of these attachments are bonds and the other two are lone pairs. Therefore, the resulting molecu… - What is the molecular geometry of BF3, boron trifluoride?
If we drew the electron dot structure for BF3, boron trifluoride, we will notice that there are three attachment point, and 3 bonds, to the central atom, boron. Based on the chart, the molecular geometry for BF3would be trigonal planar, with an angle of 120 degrees between the bonds.