What is the temperature when water boils in Kelvin?
Water boils at a temperature of 100° C, which is 373.15 kelvins (or 212° F). Notice how we don't say "degrees Kelvin" like we would say "degrees Celsius" or "degrees Fahrenheit".
What is the normal freezing point of water in Kelvin?
Summary
- The freezing point for pure liquid water is not 0°?
- At 4 0 C water exhibits strange behaviour?
- water can coexists as a liquid, gas and solid phase?
- The freezing point can be changed?
- Which point of the water is considered an internationally accepted point for the Absolute Temperature scale?
- Ice can be evaporated without melting it?
What is the melting and freezing point of water in Kelvin?
Boiling, Freezing, Melting point of water in kelvin 0°F = −17.78°C (1°C +273.15) = 274.15 k (10°C +273.15) = 283.15 k 0 k = −273.15°C
How do you calculate a normal boiling point?
To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure equal to standard pressure. At whatever temperature that line intersects the vapor pressure curve of a liquid is the boiling point of that liquid.
What is boiling of water in Kelvin?
The Kelvin degree is the same size as the Celsius degree; hence the two reference temperatures for Celsius, the freezing point of water (0°C), and the boiling point of water (100°C), correspond to 273.15°K and 373.15°K, respectively.
Does water boil at 273 Kelvin?
There are three common temperature scales: Kelvin, Celsius, and Fahrenheit . Water freezes at temperatures below 273 kelvins. Water boils at 373 kelvins. …Jan 2, 2022
What is the boiling point of water in Kelvin at 1 atm?
The boiling point of water at 1 atm pressure in Kelvin scale is 373 K.
What is the boiling point of water in Kelvin Class 9?
373.2K.The boiling point of water on the Kelvin scale is 373.2K.
Is 273 Kelvin hot or cold?
Water freezes at temperatures below 273 kelvins. Water boils at 373 kelvins. Zero on the Kelvin scale is at absolute zero, the coldest possible temperature.May 5, 2010
Is 1 Kelvin hot or cold?
1 degree Kelvin is not hot at all, and in fact is nearly as cold as it is possible to be in our universe.
What is the boiling point of oxygen in Kelvin?
90.188 KelvinPeriodic Table of the ElementsNameWeightBoiling PointOxygen15.999490.188 KelvinFluorine18.998485 KelvinNeon20.179727.1 KelvinSodium22.989771156 Kelvin89 more rows
What is the boiling point at pressure 1 atm known as?
Therefore there is a set pressure known as a standard atmosphere (atm) which determines what is called the normal boiling point of a liquid. For example, water's normal boiling point is 100oC, which occurs when the atmospheric pressure is exactly 1 atm.Sep 27, 2021
How many kelvins separate the freezing and boiling points of water?
The freezing point of water on the Kelvin scale is 273.15 K, while the boiling point is 373.15 K.
What is boiling point write the boiling point of water?
At sea level, water boils at 100° C (212° F).
What is the boiling point of water?
212°F (100°C)Water / Boiling point
What is the boiling point of water in Celsius and Kelvin scale?
Boiling point of water is 100oC which is in Kelvin scale ([K]=[oC]+273.
What is the boiling point of milk?
Since milk has a lot of water, it has the same boiling point as water, approximately 100° C (depending on the atmospheric pressure); Celsius is part of the metric system, which has been adopted by different countries worldwide.
Why is the boiling point of water low?
The boiling point is low if the vapor pressure is high because less energy is necessary for a liquid to match the gas’ pressure above it as it’s being heated. 3. Temperature.
Why does water boil at 212°C?
The “thin” air has lower pressure and oxygen because of the effect of gravity. At sea level, water usually boils at 212°F (100C°). However, at higher altitudes and lower atmospheric pressures, water boils at a lower temperature. For every increase in altitude of 500 feet, the water boiling point decreases by under 1°F.
What is the temperature scale?
When reporting temperature measurement, people usually use Celsius (degree Celsius) or Fahrenheit (degree Fahrenheit). However, when reporting using the Kelvin, we don’t say “degree Kelvin.”. One of William Thomson’s best-known contributions—also known as Lord Kelvin and 1st Baron Kelvin—is the absolute temperature scale (measured in Kelvin).
What unit is used to measure temperature?
However, when reporting a temperature measurement using the Kelvin unit, we don’t say “degree Kelvin.”. Again, the boiling point of water in whatever unit (Celsius, Kelvin, and Fahrenheit) is influenced by many factors. Atmospheric pressure, vapor pressure, and temperature are three of the main ones.
Why doesn't water boil?
If the pressure is high, it will be difficult for water to turn into vapor and form bubbles. Thus, water doesn’t boil. However, if this pressure is decreased, less heat energy is necessary to move water into the vapor phase. Plus, water can boil at a lower temperature.
What is the force applied against a liquid's surface?
As defined earlier, atmospheric pressure or air pressure is the force applied against a liquid’s surface. As a thumb rule, the higher the atmospheric pressure, the more heat energy is necessary to boil a liquid—or in this case, water. This, in turn, raises the boiling point.
Standard Boiling Point of Water
The boiling point of a substance at standard atmospheric pressure is called its standard boiling point. Therefore, we can now say that the standard boiling point of water is 100 ∘ C.
The Factors Affecting Boiling Point or Boiling Point of Water
The various factors affecting the boiling point of water are discussed below.
Calculation of Boiling Point
During the conversion of a liquid into its vapours (during boiling), the heat of vapourization (It is the energy required to transform a given quantity of a substance from a liquid into a gas at a given pressure) is involved.
Presence of a Salt and Boiling Point of Water
Liquid water molecules escape and enter into their gaseous phase while boiling. But if salt is present in water, it makes it harder for the water molecules to escape and enter the gas phase. This increases the boiling point or boiling time of water. Therefore, we can say that saltwater will have a higher boiling point than pure water.
Summary
We have now understood that the boiling point is the temperature at which liquid boils. It is also defined as the temperature at which the vapour pressure of a liquid becomes equal to the prevailing atmospheric pressure. We have also learnt about the boiling point of water.
Frequently Asked Questions
We have provided some frequently asked questions on Boiling Point of Water here:
What is the boiling point of water?
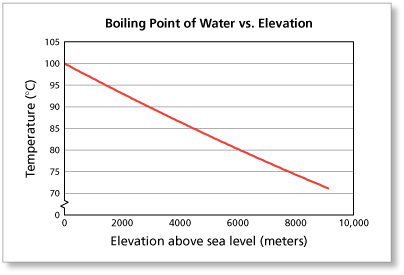
The simple answer to this question is that the boiling point of water is 100 °C or 212 °F at 1 atmosphere of pressure ( sea level ). However, the value is not a constant. The boiling point of water depends on the atmospheric pressure, which changes according to elevation.
Why does water boil at a lower temperature?
Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain), and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). The boiling point of water also depends on the purity of the water.
class 5
The Fish Tale Across the Wall Tenths and HundredthsParts and Whole Can you see the Pattern?
class 9
Circles Coordinate Geometry What is Democracy? Why Democracy?Nazism and the Rise of Hitler Socialism in Europe and the Russian Revolution
Answer
373.15 Kelvin is the value of boiling point of water on Kelvin scale of temperature.
New questions in Science
1/How many groups can appliances be placed into? (A) 4 (B) 6 (C) 2 (D) 3 2/ Labor saving device is also known as (A) kitchen tools (B) household items … (C) household equipment & tools (D) Electrical appliances 3/ When using soft furnishing we should first (A) enhance the design of the rest of the furniture or other accessories (B) measure the window length and width to make certain that the curtain is not too short.
Standard Boiling Point of Water
- The boiling point of a substance at standard atmospheric pressure is called its standard boiling point. Therefore, we can now say that the standard boiling point of water is \(100\,^\circ {\rm{C}}.\) The standard boiling point of water in the Kelvin scale is \(373.15\;{\rm{K}}.\) Generally, it is said that the boiling point of water is high. This i...
Calculation of Boiling Point
- During the conversion of a liquid into its vapours (during boiling), the heat of vapourisation (It is the energy required to transform a given quantity of a substance from a liquid into a gas at a given pressure) is involved. Therefore, if we know the heat of vaporisation and the vapor pressure of a liquid at a certain temperature, we can calculate the boiling point of a liquid. That is, \({{\rm{T}}_{…
Presence of A Salt and Boiling Point of Water
- Liquid water molecules escape and enter into their gaseous phase while boiling. But if salt is present in water, it makes it harder for the water molecules to escape and enter the gas phase. This increases the boiling point or boiling time of water. Therefore, we can say that saltwater will have a higher boiling point than pure water. Note: The presence of sugar, salt or other non-volatil…
Summary
- We have now understood that the boiling point is the temperature at which liquid boils. It is also defined as the temperature at which the vapour pressure of a liquid becomes equal to the prevailing atmospheric pressure. We have also learnt about the boiling point of water. That is, at standard atmospheric pressure conditions, water will boil at \(100\;^\circ {\rm{C}}.\) The major f…
Frequently Asked Questions
- We have provided some frequently asked questions on Boiling Point of Water here: Q1. What are the freezing and boiling points of water? Ans:The freezing point of water is \(0\,^\circ {\rm{C}}\left( {32\,^\circ {\rm{F}}} \right)\), and the boiling point of water is \(100\,^\circ {\rm{C}}\left( {212\,^\circ {\rm{F}}} \right).\) Q2. How does salt affect the boiling point of water? …