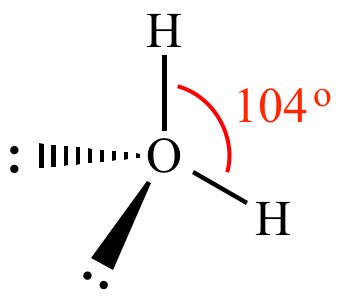Molecular Geometry Example
| Geometry | Type | # of Electron Pairs | Ideal Bond Angle | Examples |
| linear | AB 2 | 2 | 180° | BeCl 2 |
| trigonal planar | AB 3 | 3 | 120° | BF 3 |
| tetrahedral | AB 4 | 4 | 109.5° | CH 4 |
| trigonal bipyramidal | AB 5 | 5 | 90°, 120° | PCl 5 |
What are the approximate bond angles?
How do you find the bond angle?
- Write the Lewis dot structure for the molecule. Assume that you must determine the bond angles in BF3 . …
- Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule. …
- Use the VSEPR shape to determine the angles between the electron domains.
How to calculate bond angles?
Bond Angle between Bond pair and Lone pair of electrons in terms of p character Solution
- Convert Input (s) to Base Unit
- Evaluate Formula
- Convert Result to Output's Unit
How do you determine the bond angle?
Chem help: how do I determine the bond angle of a molecule?
- Get the group number of the primary element
- If its a negative charge, add that to the group number from 1) or subtract the positive charge
- Add the bonded pairs
- Divide by 2 to get the bonded pairs
- Now subtract the bonded pairs by the number of bonds to find the loan pairs.
How do you predict bond angles?
How do you predict bond angles? 1 Answer. Write the Lewis dot structure for the molecule. Assume that you must determine the bond angles in BF3.; Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule.; Use the VSEPR shape to determine the angles between the electron domains.; What is the value of the bond angles in bf3 BF 3?
Why is 109.5 The ideal bond angle?
VSEPR theory predicts methane is a perfect tetrahedron with all H-C-H bond angles equal at 109.5o, because the hydrogen atoms repel equally, and because this geometry puts the greatest distance between all four bonded electrons pairs.
What is an ideal bond?
Ideal bond angles are the angles formed from the valence shell electron repulsion (VSEPR) theory. This theory states that the lone pair and bonding pair electrons want to be as far away from each other as possible in molecules.
What is the ideal bond angle for Bent?
VSEPR NotationName of Molecular Geometry (Shape)Bond AngleAB2E1bent<120°AB4tetrahedral109.5°AB3E1trigonal pyramidal<109.5°AB2E2bent<109.5°2 more rows
What is the ideal bond angle of alkane?
These bond angles ought to be 109 degrees. The angles in an equilateral triangle are actually 60 degrees, about half as large as the optimum angle.
What is idealized bond angle deviation?
Bond angles will deviate from their ideal values according to the rule that lone pairs repel other electrons more strongly than bonding pairs. Although lone pairs are clearly smaller than atoms, they need to be closer to the nucleus of an atom than a bonding pair.
What is ideal geometry?
Ideal geometry is geometry in abstract, set apart from the physical. It is the geometry of school mathematics lessons. Its elements are the straight line, the circle, the square, the triangle… and their three-dimensional forms: the plane, sphere, cube, cone, tetrahedron, pyramid….
What is the bond angle for water?
104.5°The actual bond angle in the water molecule is 104.5°.
Is bent and angular the same?
In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped.
What is the bond angle of tetrahedral?
109.5 degreesThe proof that a bond angle in a tetrahedral molecule is 109.5 degrees is more complex than it first appears.
What is the bond angle of alkene?
approximately 120°Alkenes are hydrocarbons with C=C bonds and alkynes are hydrocarbons with C C bonds. Since C=C bonds have sp2 hybridized C, atoms or groups directly attached to a C=C bond lie in a plane and are separated by approximately 120° bond angles.
Which has a minimum bond angle?
Hence, the minimum bond angle is present in H2S.
What is bond angle of nh3?
The bond angle of ammonia is 107°.
What is the bond angle of a trigonal planer?
We know if you're AX2, then you're linear, so your ideal bond angle is 180 degrees. If your trigonal planer, it's 120. If you're tetrahedral, it's 109.5. If you're trigonal bipyramidal or you're trigonal bipyramidal, you have all of these angles involved. Then, if you're octahedral, you have 90 and 180.
Why does water have a smaller bond angle?
Water, now we have an additional bond angle, getting smaller, because now there are two lone pairs pushing away. What you're supposed to take from this is those ideal bond angles are only if the central element has no lone pairs. Once the central element starts to have lone pairs the bond is going to get smaller and smaller.
What is the bond between oxygen and hydrogen?
Two of these regions contain a pair of electrons forming a covalent bond between oxygen and hydrogen; the other two regions contain an unshared electron pair.
How to describe the geometry of a water molecule?
To describe the geometry of the water molecule, remember that the geometry of a molecule describes only the geometric relationships between its atoms. The three atoms of a water molecule are in a bent line like those of sulfur dioxide. We say the water molecule is bent.
Which molecule has a central atom that is bonded to four other atoms?
A molecule whose central atom is bonded to four other atoms is tetrahedral. One in which the central atom has one unshared pair of electrons and bonds to three other atoms will be pyramidal, and one in which the central atom has two unshared pairs of electrons and bonds to two other atoms will be bent.
How to predict how electrons will repel each other?
One way to predict the way electrons within atoms will repel each other is to apply the VSEPR (valence-shell electron-pair repulsion) model. VSEPR can be used to determine a molecule's general geometry.
What are the molecular model sets?
Most molecular model sets include the proper bond angles for atoms so you can see the molecular geometry of molecules when you make them . Grzegorz Tomasiuk / EyeEm / Getty Images
Can you use Lewis structures to predict molecular geometry?
You can use Lewis structures to predict molecular geometry, but it's best to verify these predictions experimentally. Several analytical methods can be used to image molecules and learn about their vibrational and rotational absorbance.