What is the Beer–Lambert law?
A demonstration of the Beer–Lambert law: green laser light in a solution of Rhodamine 6B. The beam radiant power becomes weaker as it passes through solution.
Can Beer Lambert law be expressed in terms of attenuation coefficient?
Expression with attenuation coefficient The Beer–Lambert law can be expressed in terms of attenuation coefficient, but in this case is better called Lambert's law since amount concentration, from Beer's law, is hidden inside the attenuation coefficient. The (Napierian) attenuation coefficient and the decadic attenuation coefficient
What is the Beer-Lambert law for the atmosphere?
The beer-lambert law for the atmosphere is written as: the optical depth for a slant path is τ′ = mτ, where T refers to a vertical path, m represents the relative air mass and a plane parallel atmosphere is given as m=sec θ where θ represents the zenith angle corresponds to the given path.
How do you use Beer-Lambert law in chemical analysis?
Chemical analysis by spectrophotometry. Beer–Lambert law can be applied to the analysis of a mixture by spectrophotometry, without the need for extensive pre-processing of the sample. An example is the determination of bilirubin in blood plasma samples. The spectrum of pure bilirubin is known, so the molar attenuation coefficient ε is known.
What is e in beer Lambert equation?
The Beer-Lambert law is expressed as: A = εLc. where, A is the amount of light absorbed for a particular wavelength by the sample. ε is the molar extinction coefficient.
What is e in absorbance?
e = A / bc. In words, this relationship can be stated as "e is a measure of the amount of light absorbed per unit concentration". Molar absorbtivity is a constant for a particular substance, so if the concentration of the solution is halved so is the absorbance, which is exactly what you would expect.
What is ε in chemistry?
molar extinction coefficient. The term molar extinction coefficient (ε) is a measure of how strongly a chemical species or substance absorbs light at a particular wavelength. It is an intrinsic property of chemical species that is dependent upon their chemical composition and structure.
What does L stand for in Beer-Lambert law?
Mathematical statement of Beer's law is A = εlc, where: A = absorption; ε = molar attenuation coefficient, l = path length (the thickness of the solution), and c = concentration of the solution. A demonstration of Beer's Law.
Why is e important in Beer's law?
Question : What is the significance of the molar absorptivity, e ? In words, this relationship can be stated as "e is a measure of the amount of light absorbed per unit concentration".
How do you calculate E from absorbance?
Here is an example of directly using the Beer's Law Equation (Absorbance = e L c) when you were given the molar absorptivity constant (or molar extinction coefficient). In this equation, e is the molar extinction coefficient. L is the path length of the cell holder....Concentration (M)Absorbances0.400.550.500.692 more rows
What is epsilon used for?
it is used to represent dual numbers: a + bε, with ε2 = 0 and ε ≠ 0. it is sometimes used to denote the Heaviside step function. in set theory, the epsilon numbers are ordinal numbers that satisfy the fixed point ε = ωε.
What is the epsilon value?
The EPSILON property has a value of approximately 2.2204460492503130808472633361816E-16 , or 2^-52 .
What are the units of molar absorptivity ε )?
The molar absorptivity is a Beer-Lambert absorption coefficient. SI unit: m2 mol-1."
How do you find the Epsilon in Beer's law?
All Answers (2) Dear Jayalakshmi Arunkumar, The equation to be used (Beer-Lambert Law) is: A = E l C ; where A is the absorbance; C is the concentration and l is the cell's width, E (epsilon coefficient) and its unit is mol/dm3.
What is AMAX chemistry?
Illustrated Glossary of Organic Chemistry - Lambda max. Lambda max (λmax): The wavelength at which a substance has its strongest photon absorption (highest point along the spectrum's y-axis). This ultraviolet-visible spectrum for lycopene has λmax = 471 nm.
How do you find t from absorbance?
To convert a value from absorbance to percent transmittance, use the following equation:%T = antilog (2 – absorbance)Example: convert an absorbance of 0.505 to %T:antilog (2 – 0.505) = 31.3 %T.
Why is the Beer-Lambert law called the Beer-Lambert law?
The reason for so many names is because more than one law is involved in it. In 1729 Pierre Bouger discovered the law and published it in Essai d’optique sur la gradation de la lumiere. In 1760 Lambert quoted the Bouger’s discovery in his Photometria which states that the absorbance of a sample is directly proportional to the path length of light. Lambert did not claim any discovery, but he was often credited with it. In 1852, August Beer discovered that absorbance is proportional to the sample concentration. Generally, beers law relates only to concentration while Beer-Lambert law relates absorbance to both concentration and thickness of a sample.
Why is Beer Lambert law considered a limiting law?
Presently, the Beer lambert law is declared as a limiting law because the absorbance is only nearly linear depending on the concentration. This is the reason that the attenuation coefficient also depends on concentration and density even if there are no interactions.
What did Lambert discover about the law of absorbance?
Lambert did not claim any discovery, but he was often credited with it. In 1852, August Beer discovered that absorbance is proportional to the sample concentration. Generally, beers law relates only to concentration while Beer-Lambert law relates absorbance to both concentration and thickness of a sample.
What is the deviation of Beer Lamberts law?
The law also deviates if non-monochromatic light is used. The change in temperature also leads to the deviation of Beer-lamberts’ law. The deviation may also occur if the width of the instrument is not proper.
Why is Beer's law important?
In chemistry Beers law is used to measure the concentration of chemical solutions , oxidation analysis and to measure the degradation of the polymer. Beer’s law also describes the attenuation of radiation through the Earth’s atmosphere.
What is the law of attenuation of solar radiation?
The attenuation of solar or stellar radiation is also described with the help of this law as it travels through the atmosphere. In this case, there is a scattering of radiation as well as absorption. The beer-lambert law for the atmosphere is written as:
What is Beer-Lambert Law?
The Beer-Lambert law, also known as the Beer-Lambert–Bouguer law, or the Beer's law, states that the absorbance of a solution is proportional to its concentration, absorption coefficient, molar, and optical coefficient.
Derivation and Mathematical Expression
ε denotes molar extinction coefficient or molar absorptivity (or absorption coefficient),
Applications of Beer-Lambert Law
The law finds application in analytical chemistry. Analytical chemistry is concerned with the separation, measurement, and identification of matter by the use of spectrophotometry. To obtain the results, no extensive pre-processing of the material is required.
Limitation of Beer-Lambert law
When the concentration is low, i.e. 10mM, it is simple to analyze the absorptivity coefficient of the sample using this rule, but as the concentration rises, i.e. >10mM, there is a deviation due to the increase of the electrostatic interactions.
Things to Remember
The Beer-Lambert law states that the absorbance of a solution is proportional to its concentration, absorption coefficient, molar, and optical coefficient.
Sample Questions
Ques. What role does absorbance play in determining a solution's concentration? 1 mark
What is Beer Lambert law?
Beer Lambert law is one of the popular topics in analytical chemistry. It relates the weakening of the intensity of the light to the characteristics of the medium through which it is traveling. Let’s say, we have a clear sample of a drug with a polished surface around its container. Now, passing electromagnetic radiation (incident radiation ...
What is Lambert's law?
Lambert’s Law. When monochromatic radiation (it can be UV rays) is passed through a medium, the intensity of the transmitted radiation decreases with the increase in the thickness of the absorbing medium, and it varies directly with the incident radiation. Mathematically, we can express this statement as:
What law states the linear relationship between the absorbance and the concentration of a solution sample?
Answer: In electromagnetic spectroscopy, we find many applications on Beer-Lambert’s law . This law states the linear relationship between the absorbance and the concentration of a solution sample, which enables us to determine the molar concentration of any number of solutions.
What is the value of absorbance?
Answer: The value of the absorbance lies between 0.1 and 1. If the absorbance of material is greater than or equal to 1.0 (too high), then we can say that the solution has a higher concentration.
What does the Beer Lambert law state?
Beer’s Law (Beer-Lambert Law): The amount of energy absorbed or transmitted by a solution is proportional to the solution’s molar absorptivity and the concentration of solute. In simple terms, a more concentrated solution absorbs more light than a more dilute solution does.
How is beer Lambert law used in spectroscopy?
The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution, which enables the concentration of a solution to be calculated by measuring its absorbance.
Why is the Beer Lambert law important?
The reason why we prefer to express the law with this equation is because absorbance is directly proportional to the other parameters, as long as the law is obeyed. … A = ebc tells us that absorbance depends on the total quantity of the absorbing compound in the light path through the cuvette.
How is beer Lambert law derived?
A is the amount of light absorbed for a particular wavelength by the sample.
What is the difference between Lambert law and beer law?
Lambert’s law stated that the loss of light intensity when it propagates in a medium is directly proportional to intensity and path length. … Beer’s law stated that the transmittance of a solution remains constant if the product of concentration and path length stays constant.
What is the basic principle of spectrophotometer?
Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle is that each compound absorbs or transmits light over a certain range of wavelength.
How do you calculate absorbance?
This can be given as Ay = -log10 (I/Io) where Ay is the absorbance of light with wavelength y and I/Io is the transmittance of the test material. Observe that absorbance is a pure number without units of measure. Absorbance is based on the ratio of two intensity measurements, so the resulting value has no units.
What is the Beer-Lambert law?
In theoretical physics, the Beer-Lambert Law is a solution to the Bhatnagar-Gross-Krook (BKG) operator, which is used in the Boltzmann equation for computational fluid dynamics.
What is the difference between Beer's Law and Beer-Lambert Law?
Technically, Beer's Law relates only to concentration, while the Beer-Lambert Law relates absorbance to both concentration and sample thickness.
Why is there so many names for beer law?
The reason there are so many names is because more than one law is involved. Basically, Pierre Bouger discovered the law in 1729 and published it in Essai D'Optique Sur La Gradation De La Lumière.
Why is Beer's law important?
Beer's Law is used in chemistry to measure the concentration of chemical solutions, to analyze oxidation, and to measure polymer degradation. The law also describes the attenuation of radiation through the Earth's atmosphere.
Who discovered the law of absorbance?
Basically, Pierre Bouger discovered the law in 1729 and published it in Essai D'Optique Sur La Gradation De La Lumière. Johann Lambert quoted Bouger's discovery in his Photometria in 1760, saying the absorbance of a sample is directly proportional to the path length of light.
What is the law of concentration?
The law states that the concentration of a chemical is directly proportional to the absorbance of a solution. The relation may be used to determine the concentration of a chemical species in a solution using a colorimeter or spectrophotometer. The relation is most often used in UV-visible absorption spectroscopy.
What is the application of the Beer Lamberts law?
Answer 1: The application of the Beer-Lamberts law takes place to the analysis of a mixture by spectrophotometry. Furthermore, this application is without the need for extensive sample pre-processing. The Beer-Lambert law example includes the determination of bilirubin in blood plasma samples.
Why is Beer Lambert law inaccurate?
This is because the analyte’s molecules exhibit stronger electrostatics and intermolecular interactions.
What law states that light absorption is directly proportional to the concentration of a substance?
Beer Lambert law tells us that the absorption of a quantity of light by a substance that is dissolved in a fully transmitting solvent happens to be directly proportional to the substance’s concentration and the path length of the light via the solution.
How to measure Beer Lambert law?
It is possible to measure the Beer Lambert law by calculating the concentration of a solution by making use of the absorbancies. Another way is to plot a graph of various concentrations and then align them according to their appropriate or correct absorbencies. Afterwards, one must use a colourimeter to calculate the concentration ...
How does Beer Lambert law derivation take place?
Beer-Lambert law derivation can take place from an approximation for the absorption coefficient for a molecule. This happens by carrying out an approximation of the molecule by an opaque disk whose cross-sectional area is representative of the effective area seen by a frequency w photon.
Who developed the law of concentration?
The development of the law first took place by Pierre Bouguer before 1729. After its attribution to Johann Heinrich Lambert, the law included path length as a variable that had an effect on absorbance. ...
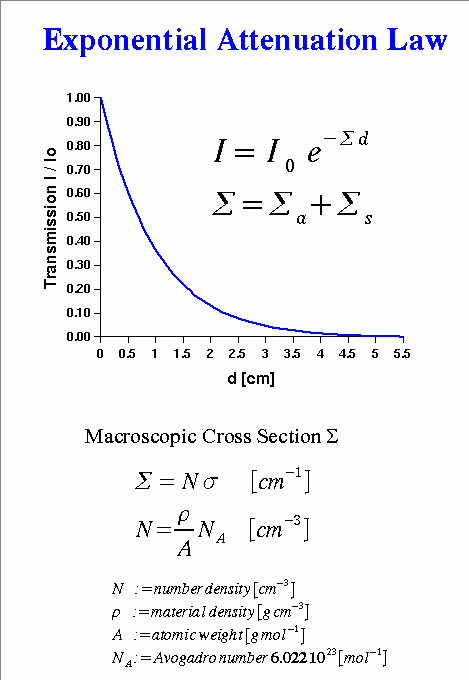
Overview
The Beer–Lambert law, also known as Beer's law, the Lambert–Beer law, or the Beer–Lambert–Bouguer law relates the attenuation of light to the properties of the material through which the light is travelling. The law is commonly applied to chemical analysis measurements and used in understanding attenuation in physical optics, for photons, neutrons, or rarefied gases. In mathematical physics, …
History
The law was discovered by Pierre Bouguer before 1729, while looking at red wine, during a brief vacation in Alentejo, Portugal. It is often attributed to Johann Heinrich Lambert, who cited Bouguer's Essai d'optique sur la gradation de la lumière (Claude Jombert, Paris, 1729)—and even quoted from it—in his Photometria in 1760. Lambert's law stated that the loss of light intensity when it propagates in a medium is directly proportional to intensity and path length. Much later, August …
Mathematical formulation
A common and practical expression of the Beer–Lambert law relates the optical attenuation of a physical material containing a single attenuating species of uniform concentration to the optical path length through the sample and absorptivity of the species. This expression is:
• is the absorbance
• is the molar attenuation coefficient or absorptivity of the attenuating species
Validity
Under certain conditions the Beer–Lambert law fails to maintain a linear relationship between attenuation and concentration of analyte. These deviations are classified into three categories:
1. Real—fundamental deviations due to the limitations of the law itself.
2. Chemical—deviations observed due to specific chemical species of the sample which is being analyzed.
Chemical analysis by spectrophotometry
The Beer–Lambert law can be applied to the analysis of a mixture by spectrophotometry, without the need for extensive pre-processing of the sample. An example is the determination of bilirubin in blood plasma samples. The spectrum of pure bilirubin is known, so the molar attenuation coefficient ε is known. Measurements of decadic attenuation coefficient μ10 are made at one wavelength λ that is nearly unique for bilirubin and at a second wavelength in order to correct fo…
Application for the atmosphere
This law is also applied to describe the attenuation of solar or stellar radiation as it travels through the atmosphere. In this case, there is scattering of radiation as well as absorption. The optical depth for a slant path is τ′ = mτ, where τ refers to a vertical path, m is called the relative airmass, and for a plane-parallel atmosphere it is determined as m = sec θ where θ is the zenith angle corresponding to the given path. The Beer–Lambert law for the atmosphere is usually writt…
See also
• Applied spectroscopy
• Atomic absorption spectroscopy
• Absorption spectroscopy
• Cavity ring-down spectroscopy
External links
• Beer–Lambert Law Calculator
• Beer–Lambert Law Simpler Explanation
Beer-Lambert Law Definition
- [Click Here for Sample Questions] Beer-Lambert law states that the absorbance of a solution is proportional to its concentration, molar absorption coefficient, and optical coefficient. It is also known as the Beer-Lambert–Bouguer law, or the Beer's law. The law states that when monochromatic light passes through a homogeneous medium, the intensity of the transmitted r…
Beer-Lambert Law Equation
- [Click Here for Sample Questions] The Beer-Lambert law equation is: Where, 1. I = Intensity 2. Io = Initial Intensity 3. μ = Coefficient of absorption 4. x= Depth (meter)
Beer-Lambert Law Derivation
- [Click Here for Sample Questions] Beer-Lambert law derivation can be derived by the following steps: The Beer-Lambert law equation is Where, 1. A denotes absorbance (no units) 2. ε denotes molar extinction coefficient or molar absorptivity (or absorption coefficient) 3. L denotes path length 4. c denotes concentration Transmittance and Absorbance T...
Applications of Beer-Lambert Law
- [Click Here for Sample Questions] Beer-Lambert Law finds application in the field of analytical chemistry. They can be described as follows: 1. Spectroscopy is based on the principle of Beer-Lambert Law. 2. Analytical chemistry is concerned with the separation, measurement, and identification of matter by the use of spectrophotometry. To obtain the results, no extensive pre …
Limitations of Beer-Lambert Law
- [Click Here for Sample Questions] The limitations of Beer-Lambert law claim that only under certain situations does the Beer-Lambert law maintain linearity. 1. As the molecules of the analyte have stronger intermolecular and electrostatic interactions due to the smaller amount of space between them, the law will produce false results at high concentrations. 2. This can alter the ana…
Things to Remember
- [Click Here for Sample Questions] 1. Beer-Lambert law states that the absorbance of a solution is proportional to its concentration, absorption coefficient, molar, and optical coefficient. 2. Beer lawstates that concentration and absorbance are exactly proportional to one other. 3. Lambert's lawstates that absorbance and path length are exactly related. 4. Beer-Lambert law Equation: I = …