What is a bent structure in chemistry?
We can commonly observe a nonlinear arrangement of molecules in triatomic molecules and ions containing only main group elements. The bent structure of these molecules is a result of the presence of lone electron pairs in the central atom. The most common examples of bent molecules include water, nitrogen dioxide, CH2, etc.
What is the molecular shape of bent?
bent The molecular shape of hydrogen sulfide is bent. The central atom sulfur is bonded to two hydrogen atoms. What is the molecular shape for H2S?, The molecular geometry of H2S is bent. Another important point is the electron geometry of H2S, which is tetrahedral.
Is BF2 a bent shape in chemistry?
You have described the bonding in BF2 (-). This molecule involved a lone pair on the boron atom hence leading to its bent shape as you described. However, as said above, BF2 (+) does not have a lone pair on the boron atom, leading to just a linear shape of this cation.
What is a bent geometry in chemistry?
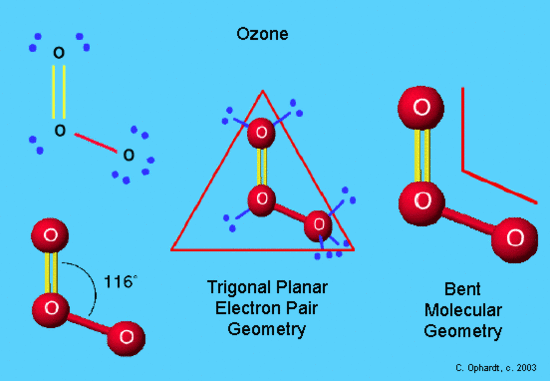
Molecule Shapes
- Angular. : Angular, bent, or v-shaped molecules contain bond angles less than 180°. ...
- Trigonal Planar. : Trigonal planar molecules form a roughly triangular shape in one plane. ...
- Tetrahedral. : A tetrahedral shape is a four-faced solid shape. ...
- Octahedral. : An octahedral shape has eight faces and bond angles of 90°. ...
- Trigonal Pyramidal. ...
What is the shape of a bent?
The shape of the orbitals is tetrahedral. Two of the orbitals contain lone pairs of electrons. The two atoms connected to the central atom form a molecule with a bent shape....Bent.Shape:bentExamples:H2O, OF2GIF File:ax2e2.gif (52K)4 more rows•Aug 21, 2020
What does bent mean in chemistry?
In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Certain atoms, such as oxygen, will almost always set their two (or more) covalent bonds in non-collinear directions due to their electron configuration.
How do you know if a molecule is bent?
You would know the molecule is bent if it has any lone pairs. In the case of water, although it has two bonds that are connected to the H, it also has two lone pairs which push down on the two bonds and make a "bent" shape rather than it being linear.
What makes a molecule bent shape?
One such shape is "bent," which occurs when there are two binding sites around the central atom, in addition to one or two lone electron pairs. Using VSEPR theory, one can determine whether or not a molecule is bent.
What is bent example?
The definition of bent is someone who is determined to take a specific course of action. An example of bent on something is someone who is determined to go to a specific college. adjective. Bent is defined as something that is not straight. An example of bent is a candy cane.
Why are atoms bent?
Bent molecules occur most frequently in group 16 elements because they have 6 electrons in their outer shell, allowing for 2 bonds and 2 lone pairs. Okay, so let's review. First, the term bent molecule refers to the geometric shape that bonded atoms have taken.
What is the difference between linear and bent?
Linear = is just a line of atoms with a 180° angle. Notice that it's 2 or 3 atoms total. Bent = Linear but bent due to the Lone Pairs that it contains, the more Lone Pairs the greater the bent and the smaller the degree.
Is a bent shape polar or nonpolar?
Are all bent molecules polar? Mostly, yes. As aforesaid, bent molecules are asymmetrical just like trigonal pyramids and that means that they are polar molecules.
How do you tell if it is linear or bent?
0:002:20Linear vs Bent - YouTubeYouTubeStart of suggested clipEnd of suggested clipAll right so let's compare linear verse bent right now linear means a line a straight line bentMoreAll right so let's compare linear verse bent right now linear means a line a straight line bent means you can't draw a straight line you have to start the line and then bend.
Is bent shape planar?
Bent or V-shaped , (trigonal/square/Pentagonal) planar, T-shaped type molecules are always Planar. A+B2 where A and B are two different elements so this type of molecule will always be Planar whatever it's hybridization is that doesn't matter.
Which hybridization is bent?
sp3 hybridizedHybridization and Electron Pair Geometry If all the bonds are in place the shape is also trigonal planar. If there are only two bonds and one lone pair of electrons holding the place where a bond would be then the shape becomes bent. For sp3 hybridized central atoms the only possible molecular geometry is tetrahedral.
Why is water molecule bent shaped?
The reason water has a bent shape is that the two lone pair of electrons are on the same side of the molecule. They really repel each other as they are only attracted to the oxygen atom in the water molecule.
Bent Shapes
Why is the structure called "bent"? How do you tell the difference between each bent molecule because there are so many of them?
Re: Bent Shapes
Structures are called bent when they are v-shaped or angular. For example, water is bent because it is non-collinear and the two lone pairs are located on the same side. Since repulsion occurs, the hydrogen bond is usually pushed down in a downward direction which causes it to be bent.
Re: Bent Shapes
Usually, when a structure is bent, it has a lone pair or more on the central atom. This lone pair adds on to the electron repulsion, pushing all the bonds away a bit more. You can tell if a molecule is bent if it has a wonky shape.
Re: Bent Shapes
When a structure is bent, it means that it is angular because the central atom has at least one lone pair of electrons. This is due to the fact that lone pairs have more repulsion, so in an molecule like water which is bent, the lone pair on the oxygen atom repel the hydrogen atoms which gives it a nonlinear shape.
Re: Bent Shapes
A structure is bent when the central molecule has two bonds and a lone pair attached. Due to the lone pair repelling the two bonds, they take an angular shape that is less than 180 degrees apart, making it look bent.
Re: Bent Shapes
The structure is bent when it has a lone pair and is bonded to two other atoms. This causes the molecule to be bent because the lone pairs have a stronger repulsion which creates the angular shape.
Re: Bent Shapes
A molecule is classified as bent because it has two atoms and at least one lone pair attached to the central atom.
Re: Bent Shape
I believe that it can either be one lone pair or two lone pairs that make a linear shape. Because a molecule with three lone pairs and two atoms would give you a linear geometry.
Re: Bent Shape
It can have one lone pair or two lone pairs. This is a link to an image showing the two different molecular shapes: http://intro.chem.okstate.edu/1314F97/C ... PMain.html
Re: Bent Shape
If a molecular had 2 bonding groups and 1 lone pair, or 2 bonding groups and 2 lone pairs, the molecular shape would be bent.
Re: Bent Shape
One or two, depending on it's VSEPR formula. It can be either AX2E or AX2E2.
Re: Bent Shape
The bent shape can have 1 lone pair when its electron geometry is trigonal planar and 2 lone pairs when its electron geometry is tetrahedral.
Re: Bent Shape
Remember with the bent shape and how it is angular, you need to know that there is a different angle degree for the different VESPER formula. AX2E2, will have an angle that is less than 120 degrees. But when it is AX2E3 the bond angle is less than 109.5 degrees.
Re: Bent Shape
Remember with the bent shape and how it is angular, you need to know that there is a different angle degree for the different VESPER formula. AX2E2, will have an angle that is less than 120 degrees. But when it is AX2E3 the bond angle is less than 109.5 degrees.