What are 4 examples of endothermic reactions?
These examples could be written as chemical reactions, but are more generally considered to be endothermic or heat-absorbing processes:Melting ice cubes.Melting solid salts.Evaporating liquid water.Converting frost to water vapor (melting, boiling, and evaporation, in general, are endothermic processes.More items...•
What are 5 examples of endothermic reactions?
Endothermic Process ExamplesMelting ice cubes.Melting solid salts.Sublimation of dry ice into carbon dioxide gas.Evaporating liquid water.Converting frost to water vapor (melting, boiling, evaporation, and sublimation are endothermic processes)Making an anhydrous salt from a hydrate.More items...•
What is endothermic reaction with example?
Endothermic Reactions A few examples of the endothermic process are photosynthesis, evaporating liquids, melting ice, dry ice, alkanes cracking, thermal decomposition, ammonium chloride in water and much more. As the name implies, 'endo' means 'to absorb,' and 'thermic' means 'heat. '
What are 2 examples of an exothermic reaction?
Here are some of the examples of exothermic reactions:Making of an Ice Cube. Making an ice cube is a process of liquid changing its state to solid. ... Snow Formation in Clouds. ... Burning of a Candle. ... Rusting of Iron. ... Burning of Sugar. ... Formation of Ion Pairs. ... Reaction of Strong Acid and Water. ... Water and Calcium Chloride.More items...
What is a simple endothermic reaction?
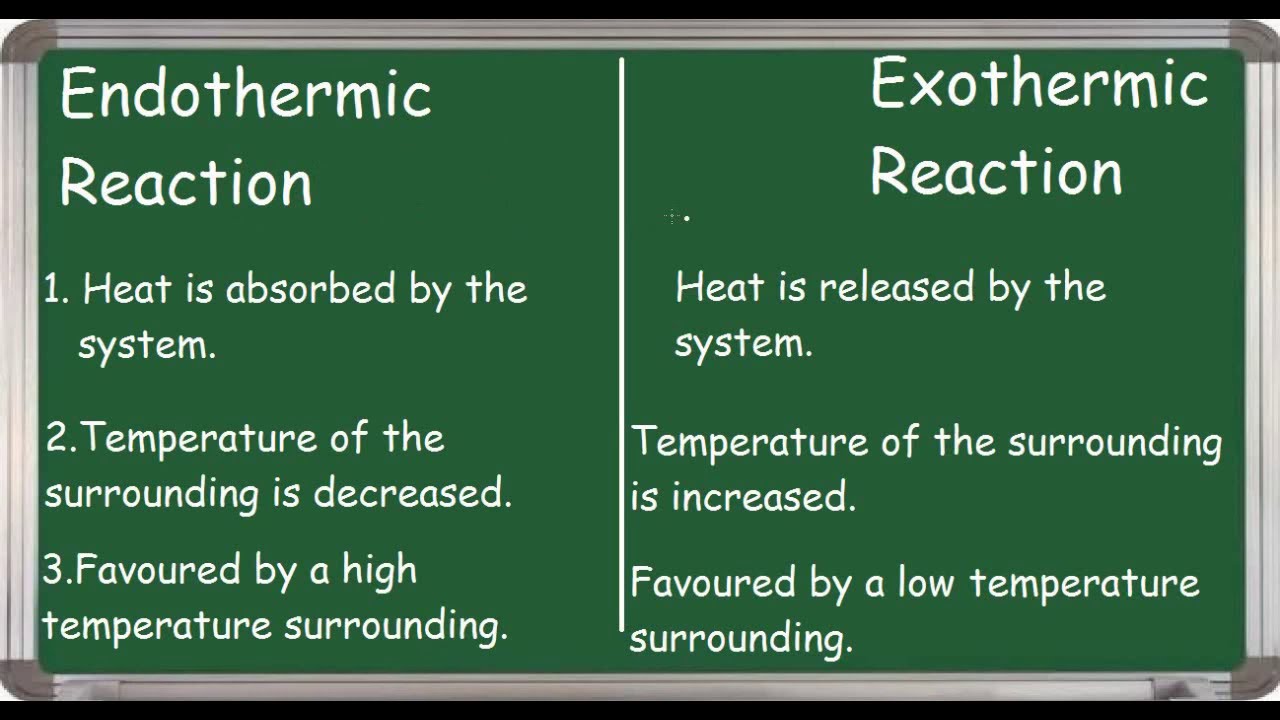
An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur.
What are endothermic reactions kids?
1:222:22Exothermic and Endothermic Reactions - Video for kids - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd do you know about thermic it means heat so endothermic reactions are those which absorb heat andMoreAnd do you know about thermic it means heat so endothermic reactions are those which absorb heat and heat is released in exothermic.
What are examples of endothermic and exothermic reactions?
Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. A good example of an endothermic reaction is photosynthesis. Combustion is an example of an exothermic reaction. The categorization of a reaction as endo- or exothermic depends on the net heat transfer.
Is freezing endothermic or exothermic?
exothermic reactionWhen water becomes a solid, it releases heat, warming up its surroundings. This makes freezing an exothermic reaction.
Which is correct for an endothermic reaction?
Solution : For an endothermic reactions is positive because in endothermic reaction heat is always absorbed. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
What are three examples of an endothermic reaction?
Melting ice cubes, melting solid salts, and sublimation of dry ice into carbon dioxide gas are three instances of endothermic reactions.
What is endothermic reaction give one example?
Endothermic reaction is a chemical reaction in which heat is absorbed. Temperatures drop as a result of it. e.g. Nitric oxide gas is generated when...
What are endothermic and exothermic reaction?
The temperature of the immediate surroundings rises as an exothermic process releases heat. An endothermic process absorbs heat from the environmen...
What type of reaction occurs in handwarmer?
In hand warmers, the chemical reaction is exothermic, which means it releases surplus energy as heat. As a result, you can warm your hands by combi...
Why self heating cans can only be used once?
Because the metal at the bottom of the can is coated with a heat-sensitive substance that reacts when it comes into touch with food or drink, the s...
What happens when you put hand warmers in water?
A hand warmer is made up of sodium acetate that has been dissolved in water. The solution has been heated to dissolve more sodium acetate, making i...
1. Explain the Differences Between Endothermic and Exothermic Reactions?
An Endothermic Reaction soaks up heat. Heat is produced by an exothermic Reaction. Endothermicity and exothermicity depend on whether there is more...
2. During the Endothermic Reaction, what form of Energy Transfer would Occur?
kinetic energy to chemical energy. Heat is absorbed in the Endothermic Reaction and then converted into chemical energy. Remember that the measure...
3. Give some examples of Endothermic Reactions.
Some of the examples of Endothermic Reactions are as follows:Photosynthesis: here, the chlorophyll in plants helps in the transformation of carbon...
4. What are Exothermic Reactions?
Exothermic Reactions can be defined as those reactions where energy is released as light or heat. In simple terms, energy is transferred into the s...
5. What are the examples of Exothermic Reactions?
We can observe exothermic Reactions in our day-to-day life, they are as follows:Formation of an ice cube: this is the process where the water chang...
6. What is the difference between Exothermic Reaction and Endothermic Reaction?
The reactions which involve energy use when the process of dissociation is taking place and a new bond is formed then it is called an Endothermic R...
7. Can I clear my Chemistry doubts on Vedantu?
Yes, you can clear your chemistry doubts on Vedantu. Vedantu has many subject experts who will make sure that your concepts are cleaned thoroughly...
What are the compounds produced by endothermic reactions?
Compounds produced by endothermic reactions have stored or potential chemical energy in their bonds , which may be released spontaneously in the event of an explosion. Chlorates, perchlorates, and nitrates contain certain compounds. This can spontaneously combust, making them dangerous to deal with whether there is any criminal intent or not.
What is the difference between endothermic and exothermic reactions?
Difference Between Endothermic and Exothermic Reactions. Chemical reactions involving the use of energy at the time of dissociation to create a new chemical bond are known as endothermic reactions, whereas exothermic reactions are those chemical reactions in which the energy is evolved or released. This is done in the form of heat.
How is heat converted into chemical energy?
Answer: kinetic energy to chemical energy. Heat is absorbed in the endothermic reaction and then converted into chemical energy. Remember that the measure of heat is the temperature, and it is not energy in itself.
How does energy absorption occur in endothermic reactions?
More energy is taken from breaking bonds in an endothermic reaction than is released to create them , so the reaction proceeds with a net energy absorption.
Why do endothermic reactions cause their environment to cool down?
They tend to cause their environments to cool down because endothermic reactions draw heat from their environments. As endothermic reactions yield higher energy products than the reactants, they are also usually non-spontaneous. The change in enthalpy is always positive for an endothermic ...
Which process requires energy in the form of heat?
There is a need for energy in the form of heat in the endothermic process, while energy in the exothermic process grows or is released.
What is an example of bond breaking?
The burning of carbon with oxygen to make carbon dioxide is an example. Bond breaking involves energy, while energy is released by bond forming. The equilibrium between the two produces a positive or negative change in energy for the reaction. Chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic, ...
What is endothermic reaction?
Endothermic Reaction Defined. Chemical reactions are all about the energy. In an endothermic reaction, heat is used for the reaction to occur. The heat energy breaks the bonds in the substance causing the reaction. As the heat is absorbed, the product will be colder.
What is the difference between endothermic and exothermic?
However, the two are different. The main difference is endothermic absorbs heat while exothermic produces heat.
Is bread an endothermic product?
Nothing is better than fresh-baked bread. In addition to smelling delicious, baking bread is an endothermic example. The flour, yeast, and other ingredients used in creating the dough are heated. They absorb the heat making chemical reactions happen. And, the product is quite tasty!
Is chemical fire exothermic or exothermic?
A chemical fire is a great exothermic example. The right, or should you say the wrong, combination can make the chemicals burst into flames. Flames give off heat. Therefore, in an exothermic reaction, heat is released and temperatures go up rather than going down.
Is cooking an egg endothermic?
You might not have realized cooking an egg was an endothermic reaction. However, energy from the pan is absorbed to cook the egg in this endothermic reaction. Try it at home! It’s a yummy example.
Do endothermic reactions cause cold?
Well, some reactions cause cold. Endothermic reactions absorb heat and lead to the product being colder. Dive into some fun examples of endothermic reactions at play. Find out how they are different from exothermic reactions through examples. endothermic reaction examples.
What is endothermic reaction?
An endothermic reaction is one which results in a net decrease in temperature because it absorbs heat from the surroundings and stores the energy in the bonds formed in the reaction .
What is an example of an endothermic reaction that occurs every day in Earth's atmosphere?
Formation of Nitric Oxide: An example of an endothermic reaction that occurs every day in Earth's atmosphere is the combination of molecular oxygen with molecular nitrogen to form nitric oxide. Chemists know exactly how much heat energy it takes for this reaction to occur. The balanced equation for this reaction is:
Is sweating an endothermic process?
An example of an endothermic process with which everyone knows is sweating, the process by which the body produces water on the skin as a cooling strategy. It works because water absorbs energy when it changes state from a liquid to a gas. This is an endothermic process, but it isn't a reaction, because a reaction always involves the destruction or formation of chemical bonds. On the other hand, squeezing an instant-cold ice pack does produce an endothermic reaction. A chemical in the pack reacts with water to absorb energy and freezes the water into ice.
Is the formation of new bonds exothermic or endothermic?
When energy is released as heat, the process is exothermic, and when heat is absorbed, the process is endothermic. ...
Is a chemical reaction endothermic or exothermic?
A reaction may involve multiple processes, and some of these may release heat, but as long as a reaction involves a net reduction in temperature, the reaction is endothermic . It's possible for this to happen because a chemical reaction always proceeds in a way that increases entropy. By contrast, exothermic reactions are those which release heat.
What is endothermic reaction?
Endothermic reactions are chemical processes in which the reactants absorb heat from the environment to be completed. These reactions cause a cooling effect by lowering the temperature of the surrounding environment. For example, water absorbs heat from a burner to be converted into vapour.
What happens when endothermic reactions occur?
Endothermic reactions produce compounds with stored chemical energy in their bonds, which could suddenly discharge in the event of an explosion. Certain chemicals are found in chlorates, perchlorates, and nitrates. This can cause them to spontaneously combust, making them risky to deal with whether or not there is criminal intent. When coupled with other materials, they can produce a high-explosive mixture.
Why are endothermic reactions non-spontaneous?
Endothermic reactions are frequently non-spontaneous because they produce more energy products than reactants. The change in enthalpy for an endothermic reaction is always positive. The products of an endothermic reaction have more energy than the reactants. As a result, the change in enthalpy is positive, and the reaction absorbs heat from the environment. Such reactions absorb heat from the atmosphere, resulting in a colder temperature in the system where the reaction occurs. Furthermore, the enthalpy, which is the difference in heat energy during the transfer of reactants to products, increases as the reaction progresses.
What does it mean when the enthalpy change is positive?
Ans. If the enthalpy change for a reaction is positive, the reaction is endothermic (endo- = in), meaning it absorbs heat as it continues.
What happens to energy in an endothermic reaction?
Ans. In an endothermic reaction, more energy is consumed in breaking bonds than is released in creating them, hence the reaction proceeds with net energy absorption.
Is heat kinetic or chemical energy?
Ans. Chemical energy to kinetic energy is the answer. In an endothermic process, heat is absorbed and subsequently transformed into chemical energy. Keep in mind that the temperature is the unit of measurement for heat, not energy.
Is ammonium nitrate endothermic?
When dissolved in water, ammonium nitrate (NH 4 NO 3 ), a key component of instant cold packs, splits into the ammonium cation (NH 4+) and the nitrate anion (NO 3– ). By interacting with the OH – and H + ions in water, these ions generate ammonium hydroxide (NH 4 OH) and nitric acid (HNO 3 ), respectively. This reaction is endothermic because it absorbs heat from the environment and cools it.