What units are used to measure atomic radius?
Apr 08, 2020 · Units used to measure atomic radius: Angstroms (Å): This the most common unit used. Equivalent to 1.0 x 10-10 meters. Nanometer (nm): Equivalent to 1.0 x …
What is the atomic radius (r)?
Jan 20, 2020 · Atomic radii are often measured in angstroms (Å), a non-SI unit: 1 Å = 1 × 10 −10 m = 100 pm. Also Know, what does atomic radius mean? The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the center of the nucleus to the boundary of the surrounding shells of electrons.
What is the equivalent of nm in atomic radius?
Aug 15, 2019 · The atomic bonds restrict the electrons and nucleus, and due to this, the ions or atoms don’t have a specific shape. The measuring unit for the ionic radius is Armstrong (A 0) or picometers (pm). The characteristic radius ranges from 30 to 200 pm.
What is the atomic radius of all the elements in order?
Units used to measure atomic radius: Angstroms (Å): This the most common unit used. Equivalent to 1.0 x 10 -10 meters. Nanometer (nm): Equivalent to 1.0 x 10 -9 meters. Picometer (pm): Equivalent to 1.0 x 10 -12 meters.
What unit is atomic radius measured in?
Typical atomic radii have values of about one or two angstrom units. (One angstrom, 1 Å, equals 10−10 metre.)
What are the units for the atomic radius quizlet?
The unit of measure for atomic radius is pm which stands for picometer. One picometer is equal to a trillionth of a meter (DANG THATS SMALL!!)
How is atomic radius measured?
The atomic radius is calculated by measuring the distance between the nuclei of two identical atoms bonded together. Half this distance is the atomic radius.Sep 25, 2021
What is atomic radius in chemistry?
Atomic radius: The radius of an atom. This distance between an atom's nucleus and outer electron shell. (This is not a fixed entity, so there are numerous definitions of this term, depending upon the measurement used.)
Where is the atomic radius on the periodic table?
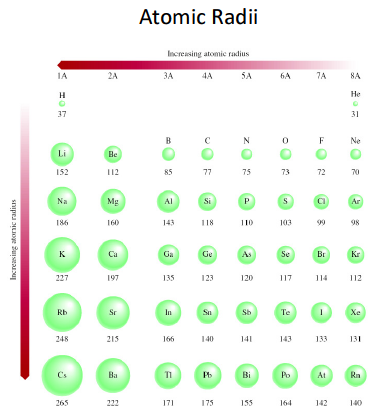
Atomic Radius Trend on the Periodic Table. Atomic radii increase toward the bottom left corner of the periodic table, with Francium having the largest atomic radius. Atoms decrease in size across the period and increase in size down the group.Jan 16, 2022
What is the unit of measurement of atomic radius Class 9?
Atomic Radius is measured in Nanometres (10-9 m).
Is atomic size and atomic radius same?
What is Atomic Size? Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. In basic chemistry, the atomic radius is defined as the shortest distance between the atom's nuclei and the outermost shell of the atom.
What is the atomic radii of hydrogen?
53 pmHydrogen / Atomic radiusExplanation: The atomic radius is the distance between the nucleus's centre and the boundary of the electrons that surround it. The atomic number of hydrogen is 1 and its standard atomic weight is 1.008. It has an atomic radius of 53 pm, which stands for picometer measurement.
Does atomic radius increase across a period?
The size of an atom generally decreases as one moves from left to right for a certain period. In general, the atomic radius decreases across a peri...
Why can’t atomic radii be measured directly?
It is difficult to determine the atomic radii because of the uncertainty in the position of the outermost electron.
What is the meaning of atomic radii?
Atomic radius is the mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons.
Which element has the largest atomic radius?
Francium has the largest atomic radius.
What is Bohr radius?
The Bohr model of the atom predicted the radius of the lowest-energy electron orbit. Bohr’s radius is only relevant to atoms and ions with a single...
What is the radii of an atom?
1. Van Der Waals Radius: Van-der-Waals radii are determined from the contact distances between unbonded atoms in touching molecules or atoms. 2. Ionic Radius: The ionic radius is the radius of an atom forming an ionic bond or an ion.
What is the measure of the size of an atom?
The measure of the size of its atoms, usually the mean or typical distance from the center of the nucleus to the boundary of the surrounding shells of electrons. This is mostly similar to the idea of the radius of a circle, where we can consider the nucleus to be the centre of the circle and the outermost orbital of the electron to be ...
What are the subatomic particles in an atom?
What is an Atom? An atom is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud. The electrons carry a negative charge and the protons carry ...
How are radii determined?
The radii of atoms are therefore determined by the bonds they form. An atom will have different radii depending on the bond it forms; so there is no fixed radius of an atom. The different radius is van der Waals radius, ionic radius, metallic radius and covalent radius.
Is the number of protons and electrons equal?
In a neutral atom the number of protons and the number of electrons are equal. Often, but not always, the number of neutrons is the same, too.
What is the atomic radius of an atom?
The atomic radius (r) of an atom can be defined as one half the distance (d) between two nuclei in a diatomic molecule. Atomic radii have been measured for elements. The units for atomic radii are picometers, equal to 10 −12 meters. As an example, the internuclear distance between the two hydrogen atoms in an H 2 molecule is measured to be 74 pm.
What is the trend of atomic radius?
Periodic Trend. The atomic radius of atoms generally decreases from left to right across a period. There are some small exceptions, such as the oxygen radius being slightly greater than the nitrogen radius. Within a period, protons are added to the nucleus as electrons are being added to the same principal energy level.
How is the size of an atom defined?
The size of an atom is defined by the edge of its orbital. However, orbital boundaries are fuzzy and in fact are variable under different conditions. In order to standardize the measurement of atomic radii, the distance between the nuclei of two identical atoms bonded together is measured.
Why is the size of an atom important?
The size of atoms is important when trying to explain the behavior of atoms or compounds. One of the ways we can express the size of atoms is with the atomic radius . This data helps us understand why some molecules fit together and why other molecules have parts that get too crowded under certain conditions.