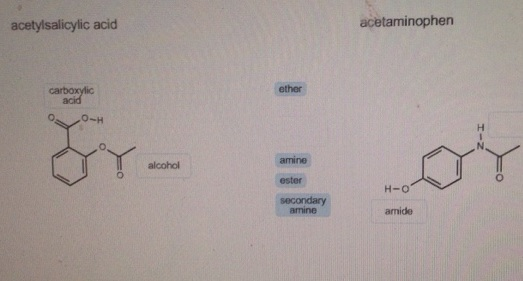
Which functional groups are present in acetaminophen?
| Aspirin Acetylsalicylic acid | Acetaminophen (neutral) | |
| Structure | ||
| Acid or base? | acid | neutral |
| Concentration in ethyl acetate when aque ... | high | high |
| Concentration in ethyl acetate when aque ... | low | high |
- The functional groups in acetaminophen are phenol, aromatic ring, and amide.
- Explanation: ...
- The structure of acetaminophen is (in figure)
- The group at the top of the molecule is a hydroxyl group. ...
- But an –OH group attached to a benzene ring has special properties. ...
- The six-membered ring is an aromatic ring.
Which are considered the functional groups in acetaminophen?
There are three functional groups found in aspirin:
- Carboxylic acid consists of a carbonyl group (CO) and a hydroxyl group (OH). It's also referred to as the R-COOH group.
- Ester consists of a carbonyl group (CO) bound to an oxygen group.
- The aromatic group (benzene) is the ring you see in aspirin.
What are functional groups does acetaminophen contain?
What Functional Groups Are Present in Acetaminophen? The functional groups present in acetaminophen are hydroxyl, abbreviated OH, amide, abbreviated HN-CO-R and the aromatic group, which is known as the benzene ring. It is an aromatic compound and a weak acid (Paracetamol).
What are the functional groups present in phenacetin?
Phenacetin
- Phenacetin. Phenacetin is group 2A (reasonably anticipated to be a human carcinogen) based on sufficient evidence of carcinogenicity in experimental animals according to the IARC Working Group noted above.
- Biotransformation. ...
- Kidney. ...
- Amines and Amides. ...
- Hypernation and Concatenation. ...
What is the daily dose of acetaminophen?
Taking more than one medicine that contains acetaminophen at a time increases your chance of taking too much and that can harm your liver. Don’t take more than 4,000 mg in 24 hours. Why? 4,000 mg is the acetaminophen dosage daily limit. Taking more increases your chance of harming your liver.
How many functional groups are there in acetaminophen?
Paracetamol (acetaminophen) contains three functional groups: hydroxyl group (OH) amide group (HN-CO-R) aromatic group (benzene ring)
Which three functional groups are all present in a molecule of paracetamol acetaminophen?
Paracetamol contains three functional groups: the hydroxyl group (OH), the amide group (HN-CO-R), and the aromatic group (benzene ring), as shown in Fig. ...
What functional groups are found in aspirin?
There are three different functional groups in aspirin, which contribute to its properties, a weak acid soluble in basic solutions. These functional groups include carboxylic acid, ester, and an aromatic group.Jan 4, 2022
What are the 5 main functional groups?
Hydroxyl, sulfhydryl, carbonyl, carboxyl, amino and phosphate groups.
What functional groups are in ketoprofen?
The four functional groups in the structure of ketoprofen drugs are:Aromatic ring- A phenyl ring of six carbon atoms joined together by alternate double and single bonds.Ketone-carbonyl carbon (C=O) is bonded between two different carbons is called ketone.Aromatic ring.More items...
How many functional groups does ibuprofen have?
Ibuprofen contains two functional groups: carboxyl group (COOH) aromatic group (benzene ring)
Is benzene ring a functional group?
Benzene ring: An aromatic functional group characterized by a ring of six carbon atoms, bonded by alternating single and double bonds. A benzene ring with a single substituent is called a phenyl group (Ph).
Are esters functional groups?
Esters are a functional group commonly encountered in organic chemistry. They are characterized by a carbon bound to three other atoms: a single bond to a carbon, a double bond to an oxygen, and a single bond to an oxygen.
What functional groups are present in thyroxine?
Functional groups: Phenolic –OH group (Ar-OH), halide (-I), ether (Ar-O-Ar), primary amine (-NH2) carboxylic acid (-COOH) groups are present in thyroxine.
What are the 7 functional groups?
Functional groups include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl.Mar 5, 2021
What are functional groups?
A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds.
What are the basic functional groups?
What are the four functional groups? In biological molecules, some of the essential functional groups include hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl groups. These groups play a significant role in forming molecules such as DNA, proteins, carbohydrates, and lipids.
What are the functional groups of acetaminophen?
The functional groups in acetaminophen are hydroxyl, aromatic ring, and amide. Click to see full answer.
What is the structure of T3D2571?
Structure for T3D2571: Acetaminophen. belongs to the class of organic compounds known as 1-hydroxy-2-unsubstituted benzenoids. These are phenols that are unsubstituted at the 2-position.
What are the functional groups of acetaminophen?
The functional groups in acetaminophen are hydroxyl, aromatic ring, and amide. Click to see full answer. Accordingly, what is the chemical structure of acetaminophen? C8H9NO2.
Does acetaminophen have an ester functional group?
Acetaminophen would give a positive phenol test when ferric chloride is added because it does contain a phenol group. Acetaminophen does not have an ester functional group, but it does have an amide group, acetanilide. Also asked, is acetaminophen acidic or basic?
How does acetaminophen release to the atmosphere?
Acetaminophen's production and use as an analgesic and the production and use in the manufacture of azo dyes and photographic chemicals may result in its release to the environment through various waste streams. If released to air, an estimated vapor pressure of 6.3X10-5 mm Hg at 25 °C indicates acetaminophen will exist in both the vapor and particulate phases in the atmosphere. Vapor-phase acetaminophen will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 22 hrs. Particulate-phase acetaminophen will be removed from the atmosphere by wet and dry deposition. Acetaminophen absorbs light at wavelengths >290 nm and, therefore, may be susceptible to direct photolysis by sunlight. If released to soil, acetaminophen is expected to have very high mobility based upon an estimated Koc of 21. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 8.8X10-10 atm-cu m/mole. Acetaminophen is not expected to volatilize from dry soil surfaces based upon its vapor pressure. The biodegradation half-lives for non-adapted, phenol -adapted, and cresol-adapted activated sludge were 21, 40, and 13 days, respectively, suggesting that biodegradation may be an important environmental fate process in soil and water. If released into water, acetaminophen is not expected to adsorb to suspended solids and sediment based upon the estimated Koc. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. An estimated BCF of 3 suggests the potential for bioconcentration in aquatic organisms is low. Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions (pH 5 to 9). Occupational exposure to acetaminophen may occur through inhalation and dermal contact with this compound at workplaces where acetaminophen is produced or used. Monitoring and use data indicate that the general population may be exposed to acetaminophen via ingestion of drinking water, and ingestion and dermal contact with this compound and other products containing acetaminophen. Exposure to acetaminophen among the general population may be widespread through use of the drug as an analgesic. (SRC)
What is acetaminophen test?
An acetaminophen test system is a device intended to measure acetaminophen, an analgestic and fever reducing drug, in serum. Measurements obtained by this device are used in the diagnosis and treatment of acetaminophen overdose.
How much of acetaminophen is bound to red blood cells?
Volume of distribution is about 0.9L/kg. 10 to 20% of the drug is bound to red blood cells. [A176357] Acetaminophen appears to be widely distributed throughout most body tissues except in fat. [Label]
What is the most common analgesic?
Acetaminophen (paracetamol), also commonly known as Tylenol, is the most commonly taken analgesic worldwide and is recommended as first-line therapy in pain conditions by the World Health Organization (WHO). It is also used for its antipyretic effects, helping to reduce fever.
How long does it take for acetaminophen to reach its peak?
[A35815] Peak blood levels of free acetaminophen are not reached until 3 hours after rectal administration of the suppository form of acetaminophen and the peak blood concentration is approximately 50% of the observed concentration after the ingestion of an equivalent oral dose (10-20 mcg/mL). [F4124] The percentage of a systemically absorbed rectal dose of acetaminophen is inconsistent, demonstrated by major differences in the bioavailability of acetaminophen after a dose administered rectally. Higher rectal doses or an increased frequency of administration may be used to attain blood concentrations of acetaminophen similar to those attained after oral acetaminophen administration. [Label]
Where is acetaminophen metabolized?
Acetaminophen is the major metabolite of _ phenacetin _ and _ acetanilid _. [F4124] Acetaminophen is mainly metabolized in the liver by first-order kinetics and its metabolism of comprised of 3 pathways: conjugation with glucuronide, conjugation with sulfate, and oxidation through the cytochrome P450 enzyme pathway, mainly CYP2E1, to produce a reactive metabolite ( N-acetyl-p-benzoquinone imine or NAPQI ). At normal therapeutic doses, NAPQI undergoes fast conjugation with glutathione and is subsequently metabolized to produce both cysteine and mercapturic acid conjugates. [Label] High doses of acetaminophen (overdoses) can lead to hepatic necrosis due to the depletion of glutathione and of binding of high levels of reactive metabolite ( NAPQI) to important parts of liver cells. The abovementioned damage to the liver can be prevented by the early administration of sulfhydryl compounds, for example, methionine and N-acetylcysteine . [A35814]
How long does acetaminophen last?
The half-life for adults is 2.5 h after an intravenous dose of 15 mg/kg. [Label] After an overdose, the half-life can range from 4 to 8 hours depending on the severity of injury to the liver, as it heavily metabolizes acetaminophen. [A35815]
What is the difference between paracetamol and Tylenol?
1 Paracetamol was marketed as Panadol by Sterling-Winthrop Co in 1953 in the UK. 2 Paracetamol was marketed as Tylenol by McNeil Laboratories in 1955 in the USA. 4 This is only a general 'rule of thumb' but it is useful because it can be difficult to determine whether a carbon atom has 'gained' or 'lost' electrons.
How to make paracetamol crystallize?
The remaining solid is impure paracetamol (acetaminophen). Step 1: Add 10 mL water to the impure paracetamol (acetaminophen) and heat gently to dissolve it. Step 3: Cool the filtrate to crystallize the paracetamol (acetaminophen).
How much paracetamol is in panadol?
Tablets such as panadol usually contain 500 mg of paracetamol (acetaminophen). Procedure to determine the paracetamol (acetaminophen) content in commercially available tablets: Separation of paracetamol (acetaminophen) from commercially available tablets:
How is paracetamol made?
Paracetamol (acetaminophen) can be prepared from phenol (hydroxybenzene) in a three step process involving: (i) nitration. (ii) reduction. (iii) formation of the amide. Paracetamol (acetaminophen) undergoes hydrolysis in acidic conditions to produce an amine and a carboxylic acid .
What is the chemical in paracetamol?
In these tablets the paracetamol has been mixed with citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid) and sodium hydrogencarbonate (sodium bicarbonate). When placed in water, the citric acid and sodium hydrogencarbonate in the tablet react to produce bubbles of carbon dioxide.
What is the reaction of an amine with anhydride?
With the exception of tertiary amines, amines undergo reaction with anhydrides to produce amides. 4-aminophenol, an amine, suspended in water at room temperature readily reacts with ethanoic anhydride (acetic anhydride) to produce a precipitate of the amide paracetamol (acetaminophen) as shown below: amine.
What is the ion that attacks the benzene ring of phenol?
The nitronium ion, NO 2+, attacks the benzene ring of phenol to produce a mixture of various structural isomers of nitrophenol. The OH (hydroxyl) functional group of phenol (hydroxybenzene) is said to activate the benzene ring at the 2- and 4- positions. This results in the formation of 2-nitrophenol and 4-nitrophenol.