What are the families of the periodic table?
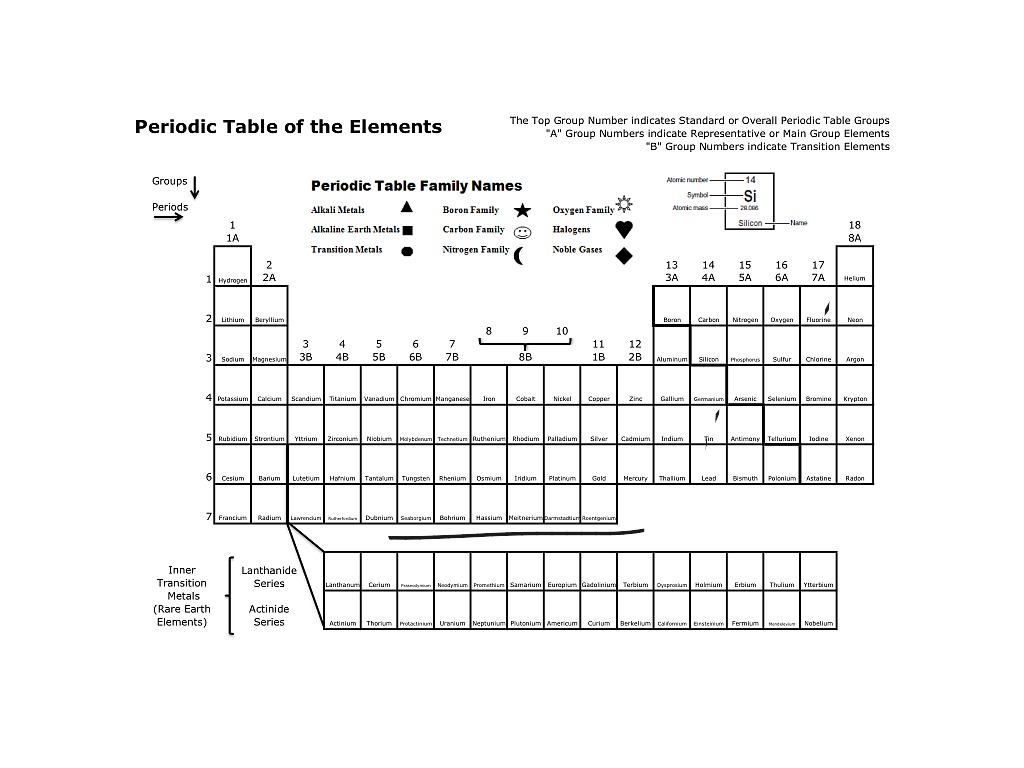
5 Element FamiliesAlkali metals.Alkaline earth metals.Transition metals.Halogens.Noble gases.
What is the 9th group in the periodic table called?
Transition MetalsGroup 9: Transition Metals.
How many families does the periodic table have?
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered.
Why are there 8 groups in the periodic table?
The idea behind the periodic system is to find regularities (hence the name) between elements. It turns out, that individual groups and trends within them are similar in the 8 "main" groups (as you have learned).
What are the 7 groups of the periodic table?
What are the 7 groups in the periodic tableThe Alkali Metals.The Alkaline Earth Metals.The Transition Metals.The Metalloids.Other Metals.The Non-metals.The Halogens.The Noble Gases.More items...
How many groups does the periodic table have?
18Groups are numbered from 1 to 18. From left to right in the periodic table, there are two groups (1 and 2) of elements in the s-block, or hydrogen block, of the periodic table; ten groups (3 through 12) in the d-block, or transition block; and six groups (13 through 18) in the p-block, or main block.
How many families are there in the periodic table?
The Periodic table can be divided into nine families of elements each having similar properties. The families include: Alkali Metals. Group 1 of the periodic table are the alkali metals. They are highly reactive and do not occur freely in nature.
What are the elements in Group 2?
Group 2 includes the alkaline earth elements. They are metallic elements and have an oxidation number of +2, making them very reactive. Groups 3 through 12 include 38 elements called transition metals. They are malleable and ductile and also conduct heat and electricity.
Which group of elements have valence electrons in more than one shell?
They have valence electrons in more than one shell and exhibit several common oxidation states. Groups 13, 14, & 15 include the other metals elements. They are malleable and ductile but are not the same as transition elements.
What are the properties of metalloids?
Metalloids are found between the metals and non-metals along a boundary. They have properties of both metals and non-metals. Some of the metalloids, such as silicon and germanium, are semi-conductors. Non-Metals. Groups 14-16 are non-metals. They are not able to conduct heat ore electricity well.
Where are lanthanide and actinide found?
These can be found in group 3 of the periodic table, and the 6th and 7th periods.
Do metals have valence electrons?
They do not exhibit a variety of oxidation states, they have valence electrons only in the outer shells. They are solid, have high density, and are opaque. Their oxidation numbers are +3, +/14, and -3. Metalloids are found between the metals and non-metals along a boundary.
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).