Is KClO3 soluble or insoluble in water?
Answer: KClO3 ( Potassium chlorate ) is Soluble in water. What is Soluble and Insoluble ? Solubility. Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent.
What is the temperature of KClO3 reaction?
This reaction occurs at a temperature of between 150-300 ° C. For this reaction manganese (IV) oxide can be the catalyst. Learn more about the chemical behaviour and importance of potassium chlorate (KClO 3) from the expert faculties at BYJU’S – India’s largest education company.
What is potassium chlorate?
Potassium chlorate, aqueous solution appears as a colorless liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
What does KCLO stand for?
Potassium Chlorate is an inorganic compound with chemical formula KClO 3. It is also known as Fekabit or Kaliumchlorat. It is very flammable when mixed with combustible materials.
See more
Is KClO3 soluble or insoluble?
0:021:39Is KClO3 Soluble or Insoluble in Water? - YouTubeYouTubeStart of suggested clipEnd of suggested clipTable they're soluble so compounds with group one elements like potassium chlorate are soluble inMoreTable they're soluble so compounds with group one elements like potassium chlorate are soluble in water.
Is potassium chlorate KClO3 soluble?
Potassium chlorate is a transparent to white salt that precipitates as well-formed, lustrous crystals which have a silky texture and are moderately soluble in water and poorly soluble in glycerol. Similar to potassium nitrate, it is not hygroscopic, making it useful as an oxidizer for pyrotechnics.
Does KClO3 dissociate in water?
0:561:55KClO3 + H2O (Potassium chlorate + Water) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSince kclo3 is soluble it'll dissolve in the water it'll dissociate break apart into those ions.MoreSince kclo3 is soluble it'll dissolve in the water it'll dissociate break apart into those ions.
Is potassium chloride soluble in water?
WaterGlycerolPotassium chloride/Soluble in
Is KClO aqueous?
Potassium hypochlorite solution is a colorless aqueous solution with a pungent irritating chlorine odor.
Is KCl a solid liquid or gas?
Definition of Potassium Chloride Potassium chloride naturally occurs as a white or colorless solid that has a powdery, crystalline appearance. Its chemical formula is KCl, consists of one potassium (K) atom and one chlorine (Cl) atom.
Does KClO3 react with water?
Solid potassium chlorate (KClO3) dissolves into water to form potassium (K+) and chlorate (ClO3-)...
Does chlorate dissociate in water?
0:451:49NaClO3 + H2O (Sodium chlorate + Water) - YouTubeYouTubeStart of suggested clipEnd of suggested clipEither way this is what happens when you put naclo3 in water it dissociates into the sodium ion andMoreEither way this is what happens when you put naclo3 in water it dissociates into the sodium ion and the chloride ion.
Is KClO3 acidic in water?
Potassium chlorate is an ionic compound that is dissociated into K+ and ClO3- ions. Neither of these ions is hydrolyzed in water so the pH should be 7. So potassium chlorate is neither an acid nor a base.
Will KCl dissociate in an aqueous solution?
0:301:19Equation for Potassium Chloride Dissolving in Water ( KCl + H2O)YouTubeStart of suggested clipEnd of suggested clipAdd it to liquid water and when we do that it dissolves it dissociates into the ions.MoreAdd it to liquid water and when we do that it dissolves it dissociates into the ions.
Is potassium water soluble?
Potassium is in most foods. It's easily absorbed by the body. Potassium salts dissolve in water (water soluble). It's found in solution as a positively charged particle (cation).
What is kclo3 in chemistry?
Potassium chlorate (KClO3) is a strong oxidizing agent that has a wide variety of uses. It is or has been a component of explosives, fireworks, safety matches, and disinfectants. As a high school or college chemistry student, you may have used it to generate oxygen in the lab.
What is KClO3 in rat kidney?
Sodium chlorate (NaClO3) and potassium chlorate (KClO3) ... were tested for potential promoting effect in two-stage rat renal carcinogenesis. Three groups of 15 male F344 rats each were given N-ethyl-N-hydroxyethylnitrosamine ( EHEN) at the level of 0.05% for the first 2 weeks during the initiation phase. Thereafter, the rats were treated orally for 25 weeks with NaClO3 (1%), KClO3 (1%), or distilled water (DW). Three other groups (controls) were treated similarly, except that DW was given in the initiation phase. All animals survived for the duration of the experiment. Renal neoplastic lesions were classified histologically as dysplastic foci (DF) and renal cell tumors (RCT). The number of these lesions per unit area, in six sections from each kidney, was determined microscopically. There were no statistically significant differences in the incidences and in the mean number of DF and RCT of the kidney between compound- and DW-treated rats initiated with EHEN. It is concluded that NaClO3 and KClO3 show no promoting effect in rat renal carcinogenesis under the conditions of this study.
How is KCl added to sodium chlorate?
Solid KCl is added to the sodium chlorate cell liquor in stoichiometric amounts. The mixture is then transferred to a crystallizer and the potassium chlorate slurry is removed ... The mother liquor is recycled to the cells, where the salt is converted to chlorate and the process is repeated.
What is potassium chlorate used for?
/VET/ In veterinary medicine, potassium chlorate is used as an oxidizing agent, antiseptic, and astringent ... . A 2 to 4% aqueous solution can be used as a weak astringent antiseptic in stomatitis and vaginitis; however, its effectiveness is questionable.
What is the most favorable course of action for a chemical?
SRP: The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational exposure or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material's impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations.
Is sodium thiosulfate a chlorate ion?
Intravenous sodium thiosulfate may inactivate the chlorate ion and has been reported to be successful in a necdotal reports. However, this treatment has not been clinically tested. Administration as a lavage fluid may potentially produce some hydrogen sulfide, and so it is contraindicated. /Chlorates/.
Is Cameo Chemicals copyrighted?
CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.
Is chlorate an oxidant?
Metal chlorates are oxidants in the presence of strong acid; liberates explosive chlorine dioxide gas; liberates chlorine dioxide and carbon dioxide by heating a moist metal chlorate and a dibasic organic acid; mixtures of perchlorates with sulfur or phosphorus are explosives [Bretherick 1979 p. 100]; mixtures of the chlorate with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979 p. 806]. A combination of finely divided aluminum with finely divided bromates (also chlorates and iodates) of barium, calcium, magnesium, potassium, sodium, or zinc can explode by heat, percussion, or friction [Mellor 2:310. 1946-47]. An explosion occurred during heating of a mixture of potassium chlorate and magnesium [Chem. Eng. News 14:451. 1936]. Gaseous ammonia, mixed with air reacts so vigorously with potassium chlorate that the reaction could become dangerous [Mellor 8:217. 1946-47]. A mixture of potassium chlorate and sodium amide explodes [Mellor 8:258. 1946-47]. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate, the mass explodes [Mellor 2:311. 1946-47]. Potassium chlorate and sulfuric acid react to cause fire and possible explosions [Mellor 2:315. 1946-47].
Is contact a liquid?
A colorless liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals. Ignites organic materials upon contact .
Is chlorine dioxide an oxidant?
Reactivity Profile. Metal chlorates are oxidants in the presence of strong acid; liberates explosive chlorine dioxide gas; liberates chlorine dioxide and carbon dioxide by heating a moist metal chlorate and a dibasic organic acid ; mixtures of perchlorates with sulfur or phosphorus are explosives [Bretherick 1979. p.
What temperature does potassium chlorate react with?
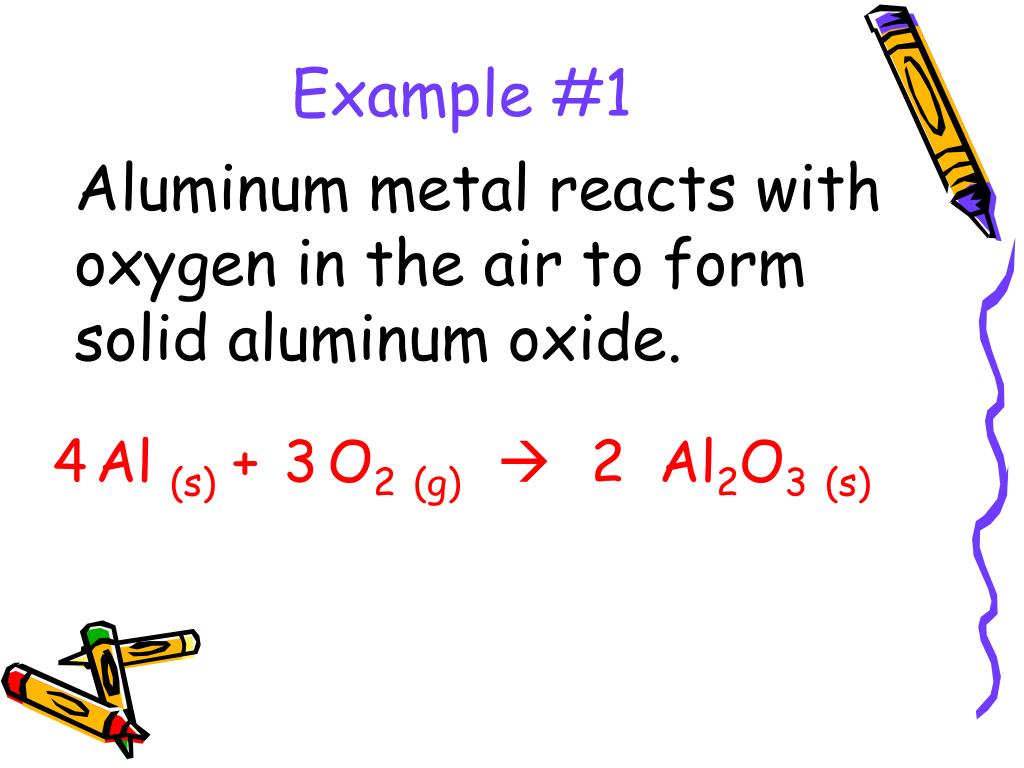
The thermal decomposition of potassium chlorate to obtain oxygen and potassium chloride. This reaction occurs at a temperature of between 150-300 ° C. For this reaction manganese (IV) oxide can be the catalyst.
What is the chemical formula for potassium chlorate?
What is Potassium Chlorate? Potassium Chlorate is an inorganic compound with chemical formula KClO 3. It is also known as Fekabit or Kaliumchlorat. It is very flammable when mixed with combustible materials. It is a compound containing potassium, oxygen, and chlorine. It appears as a white crystalline substance in its pure form.
What is potassium chlorate used for?
Uses Of Potassium Chlorate (KClO3) Potassium chlorate along with silver fulminate is used in noise-makers such as snappers and crackers. It is used as an oxidizer in smoke grenades. It is used to generate oxygen gas in college and school labs. It is used in oxygen candles or chlorate candles. It is used in limelights to supply oxygen.
Is potassium chlorate a crystalline substance?
It appears as a white crystalline substance in its pure form. It is the most widely used chlorate industry. The aqueous solution of potassium chlorate is a colourless liquid that is denser than water. It could be toxic when ingested. When it comes in contact it can irritate your eyes, skin, mucous membranes.
Is potassium chlorate thermal decomposition excessive?
Potassium chlorate thermal decomposition is not excessive, it’s just a redox reaction. Disproportionation refers to the same product that functions both as an oxidizing agent and as a reduction agent, resulting in compounds that contain the same product in different oxidation states.
What is potassium chloride?
Potassium Chloride is a metal halide composed of potassium and chloride. Potassium maintains intracellular tonicity, is required for nerve conduction, cardiac, skeletal and smooth muscle contraction, production of energy, the synthesis of nucleic acids, maintenance of blood pressure and normal renal function.
What is the intracellular concentration of potassium?
The intracellular concentration of potassium is approximately 150 to 160 mEq per liter. The normal adult plasma concentration is 3.5 to 5 mEq per liter. An active ion transport system maintains this gradient across the plasma membrane. Potassium is a normal dietary constituent and under steady-state conditions the amount of potassium absorbed from the gastrointestinal tract is equal to the amount excreted in the urine. The usual dietary intake of potassium is 50 to 100 mEq per day. Potassium depletion will occur whenever the rate of potassium loss through renal excretion and/or loss from the gastrointestinal tract exceeds the rate of potassium intake. Such depletion usually develops as a consequence of therapy with diuretics, primarily or secondary hyperaldosteronism, diabetic ketoacidosis, or inadequate replacement of potassium in patients on prolonged parenteral nutrition. Depletion can develop rapidly with severe diarrhea, especially if associated with vomiting. Potassium depletion due to these causes is usually accompanied by concomitant loss of chloride and is manifested by hypokalemia and metabolic alkalosis. Potassium depletion may produce weakness, fatigue, disturbances of cardiac rhythm (primarily ectopic beats), prominent U-waves in the electrocardiogram, and, in advanced cases, flaccid paralysis and/or impaired ability to concentrate urine. If potassium depletion associated with metabolic alkalosis cannot be managed by correcting the fundamental cause of the deficiency, e.g., where the patient requires long-term diuretic therapy, supplemental potassium in the form of high potassium food or potassium chloride may be able to restore normal potassium levels. In rare circumstances (e.g., patients with renal tubular acidosis) potassium depletion may be associated with metabolic acidosis and hyperchloremia. In such patients, potassium replacement should be accomplished with potassium salts other than the chloride, such as potassium bicarbonate, potassium citrate, potassium acetate, or potassium gluconate.
How much potassium chloride is needed for thiazide?
The oral dose of 10% potassium chloride elixir (Kay Ciel) required to reverse thiazide-induced hypokalemia was determined in 15 patients with essential hypertension who were taking Esidrix ( hydrochlorothiazide) in a dose of 50 mg. twice daily. Each patient had maintained a serum potassium concentration of at least 0.5 meq./l. less than duplicate control values (mean decreases, 0.62 meq./l.) for at least 2 months during therapy with hydrochlorothiazide, 50 mg. twice daily. Potassium chloride 10% elixir was administered in a total daily dose of 40 mg. with bimonthly increments to 60 mg., 80 mg. and 100 mg. while the thiazide administration was maintained. The serum potassium deficit was repleted to 75% in 12 of the 15 patients. In 8 of the 12, this was accomplished with 40 mg. potassium chloride daily, and in 4, with 60 mg. daily. The latter dose is recommended in patients with thiazide-induced hypokalemia.
What are the effects of potassium?
A number of neuromuscular effects can be seen, usually with potassium concentrations of 7.0 mmol/L or higher. General weakness and flaccidity precede ascending paralysis. Tremor, paresthesias, decreased vibration perception and proprioception can be seen, but the sensory function is usually intact.
Can potassium cause hyperkalemia?
The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are impaired, of if potassium is administered too rapidly intravenously, potentially fatal hyperkalemia can result. It is important to recognize that hyperkalemia is usually asymptomatic and may be manifested only by an increased serum potassium concentration (6.5-8.0 mEq/L) and characteristic electrocardiographic changes (peaking of T-waves, loss of P-wave, depression of S-T segment, and prolongation of the QT interval). Late manifestations include muscle paralysis and cardiovascular collapse from cardiac arrest (9-12 mEq/L).
Is potassium chloride reactive?
POTASSIUM CHLORIDE is not in general strongly reactive. Violent reaction with BrF3 and with a mixture of sulfuric acid potassium permanganate mixture (NTP, 1992). Reacts with concentrated sulfuric acid to generate fumes of hydrogen chloride.
Is potassium a dietary component?
Potassium is a normal dietary constituent and, under steady-state conditions, the amount of potassium absorbed from the gastrointestinal tract is equal to the amount excreted in the urine. Potassium depletion will occur whenever the rate of potassium loss through renal excretion and/or loss from the gastrointestinal tract exceeds the rate of potassium intake.