What is the difference between a compound and a mixture?
Compounds consist of elements that have been chemically bonded together, whereas mixtures contain a combination of substances not chemically bonded to one another. One key difference between compounds and mixtures is the fact that compounds will always dissolve into their individual components when placed in water, but mixtures may or may not do so depending on how they were created.
Is ammonia a pure substance?
Since ammonia is a compound, and no other substances, elements, or compounds are necessary to create ammonia, ammonia is considered to be a pure element. Is Ammonia A Mixture? No, ammonia is not a mixture. A mixture is a material composed of more than one substance, where those substances are not bonded to each other.
Is a compound always a mixture?
Mixtures are formed when elements or compounds are mixed together. properties of compounds vary from their constituent elements. mixtures show the properties of their constituent compounds or elements. mixtures components can be separated physically. compounds are always homogenous mixtures by nature.
What are some examples of compounds and mixtures?
Example of Compounds: Water (H2O), Salt (NaCl), Ammonia (NH3), Methane (CH4), Benzene (C6H6). Sugar (CHO)n, Sulphuric acid (H2SO4), Marble, Saltpetre. c)Mixture: When two or more substances are combined by physical methods in any proportion and no new substances is formed then it is called a mixture. Mixture is of two types: Homogeneous and ...
See more
Is ammonia an element?
Ammonia is a compound. It is a compound of nitrogen and hydrogen. Was this answer helpful?
Why is ammonia a compound and not a mixture?
Ammonia is formed by the combination of nitrogen and three hydrogen atoms or molecules. The combination of nitrogen and hydrogen gases gives out Ammonia gas. Therefore, Ammonia is a compound which is formed using two elements.Dec 5, 2018
Why ammonia is a mixture?
Is Ammonia A Mixture? No, ammonia is not a mixture. A mixture is a material composed of more than one substance, where those substances are not bonded to each other.
Why ammonia is a compound?
Ammonia is an inorganic compound composed of a single nitrogen atom covalently bonded to three hydrogen atoms that is an amidase inhibitor and neurotoxin.
How many atoms are in ammonia?
A triatomic molecule is a molecule made up of three atoms. They may be the same or different. As discussed above, each ammonia molecule contains four atoms. Since ammonia has more than three atoms, it is not a triatomic molecule.
What is a mixture made of?
A mixture is a material composed of more than one substance, where those substances are not bonded to each other. In the case of ammonia, the substances that make it ammonia (nitrogen and hydrogen) are bonded. They cannot easily be removed, and if they were, the remaining substance would no longer be ammonia.
How is hydrogen formed?
It is formed when two hydrogen atoms bond to each other. Since it is two atoms bonded to each other, it is a molecule. But because the atoms are the same substance, it is not considered a compound.
How are compounds and molecules formed?
A compound is formed when two different substances come together and bond to each other. A molecule is formed when any substances come together and bond to each other. In the case of a molecule, the two substances (usually atoms) can be the same, or they can be different. Either way, once the atoms form bonds to each other, it becomes a molecule.
What is pure substance?
What Is A Pure Substance? A pure substance is a substance which is composed (formed, made up of) only one kind of building block. This building block could be an element (like Gold, Silver, or Iron). This building block could also be a compound (like Salt, Sugar, or Water). Either way, a pure substance has only one of those building blocks, ...
Why is carbon dioxide not an element?
Carbon dioxide is not an element because it can be broken apart and the carbon and oxygen can be separated from each other. But if the carbons and oxygens are not bonded to each other, carbon dioxide gas does not exist. Look at gold next.
Where can I find ammonia?
You can find it naturally in our environment. In fact, the processes going on inside human bodies make ammonia. Ammonia is considered a compound. A compound is a material where two or more different substances bond together. In the case of ammonia, the substances are nitrogen and hydrogen.
What Is Carbon Monoxide?
Carbon monoxide (CO) is an odorless, colorless, toxic substance that is produced when a combustion reaction isn’t complete.
Is Carbon Monoxide an Element?
No, carbon monoxide is not an element. Carbon monoxide consists of TWO different elements i.e. Carbon and Oxygen.
Is Carbon Monoxide A Compound or Mixture?
Carbon monoxide (CO) is a compound that consists of Carbon atom and Oxygen atom that bond chemically. The ratio of Carbon Monoxide is always the same (1 Carbon and 1 Oxygen). Thus, carbon monoxide is classified as a compound, not a mixture.
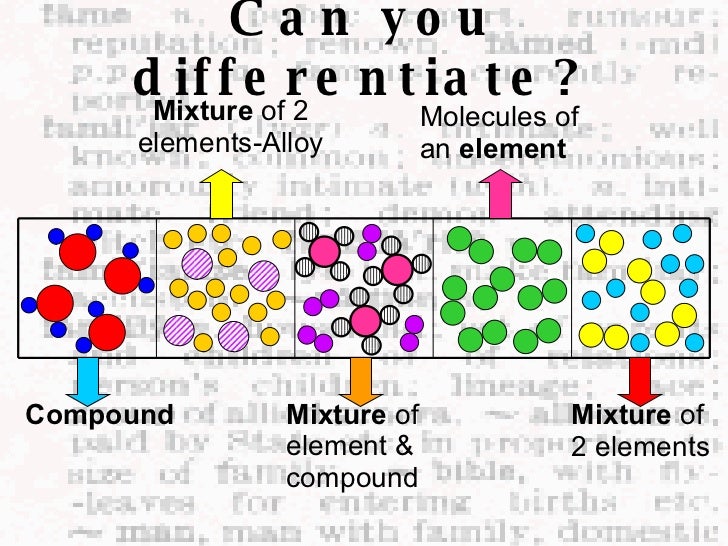
What Is An element?
What Is A compound?
- A compound is a material that consists of two or more elements that bond chemically and has a fixed ratio in composition. The bond between the elements can be ionic or covalent. Since the constituents of compound bond chemically, they can be separated through a chemical process, not a physical one. Some examples of compound or CO2 (Carbon dioxide), NaCl (Sodium chlorid…
What Is A mixture?
- A mixture in science is a material that consists of two or more elements that combine physically without making chemical bond. A mixture doesn’t have to have a fixed ratio in composition. Mixture can be categorized into homogeneous mixture and heterogeneous mixture. When a mixture is the same throughout no matter where you pick it, it’s considered homogeneous. Som…
Why Is Ammonia Not An element?
- Ammonia is not an element because it’s made of two different elements which are Nitrogen and Hydrogen, while an element is a pure substance that only made of a single type of atom. To be considered an element, a material should only be consists of purely 1 type of atom/element without any addition from other elements. Some examples of elements are Oxygen (O2), Hydrog…
Is Ammonia Organic Or inorganic?
- Ammonia (NH3) is inorganic because it does not contain the carbon atom (C). Ammonia only consists of two types of atoms which are Nitrogen (N) and Hydrogen (H). In chemistry, a compound is considered organic when it contains the bond of carbon and hydrogen (C-H). Some examples of organic compounds are salt (NaCl), water (H2O), and carbon dioxide (CO2). Inorga…
Is Ammonia Acidic Or Alkaline?
- Ammonia (NH3) is alkaline with the pH of 11, approximately. To determine whether a substance is acidic or alkaline, you can use a pH meter to test the pH. If it’s below 7, it’s acidic and if it’s above 7, it’s alkaline. In Chemistry, you can predict whether something is an acid or base by looking at the hydrogen. According to the Bronsted-Lowry theory, Something is considered an acid when it …
Conclusion
- So, ammonia (NH3) consists of two different type of atoms, which are Nitrogen and Hydrogen that combine chemically. Therefore, it’s a compound. Therefore it’s NOT an element or mixture. Ammonia is an inorganic compound since it doesn’t contain a C-H bond and it’s alkaline since it acts as a hydrogen ion acceptor.
Is Ammonia A Pure Substance?
- What Is A Pure Substance?
A pure substance is a substance which is composed (formed, made up of) only one kind of building block. This building block could be an element (like Gold, Silver, or Iron). This building block could also be a compound (like Salt, Sugar, or Water). Either way, a pure substance has on… - What is Ammonia?
Ammonia is NH3. It is a gas made up of oxygen and nitrogen atoms. You can find it naturally in our environment. In fact, the processes going on inside human bodies make ammonia. Ammonia is considered a compound. A compound is a material where two or more different substances b…
Is Ammonia A mixture?
- No, ammonia is not a mixture. A mixture is a material composed of more than one substance, where those substances are not bonded to each other. In the case of ammonia, the substances that make it ammonia (nitrogen and hydrogen) are bonded. They cannot easily be removed, and if they were, the remaining substance would no longer be ammonia. Compare a...
Is Ammonia An element?
- No, ammonia is not an element. A material can be considered an element if it cannot be broken apart into other substances. Take carbon dioxide, for example. It is made up of two oxygen atoms and one carbon atom, and these are bonded. Carbon dioxide is not an element because it can be broken apart and the carbon and oxygen can be separated from each other. But if the carbons a…
Is Ammonia A Compound Or molecule?
- Yes, as noted above, ammonia is a compound. But ammonia is also a molecule. A compound is formed when two different substances come together and bond to each other. A molecule is formed when any substances come together and bond to each other. In the case of a molecule, the two substances (usually atoms) can be the same, or they can be different. Either way, once t…
Is Ammonia A Diatomic molecule?
- No, ammonia is not a diatomic molecule. A diatomic molecule is a molecule that contains two atoms. They may be the same type of atom, or they may be different. Each ammonia molecule contains one nitrogen atom, and three hydrogens. Since it contains more than two atoms, it is not a diatomic molecule.
Is Ammonia A Triatomic molecule?
- No, ammonia is not a triatomic molecule. A triatomic molecule is a molecule made up of three atoms. They may be the same or different. As discussed above, each ammonia molecule contains four atoms. Since ammonia has more than three atoms, it is not a triatomic molecule. Interested in learning more about pure substances and mixtures? Or whether materials like bronze, copper, …