How many protons are in chlorine 39?
| Name | Chlorine |
| Atomic Mass | 35.453 atomic mass units |
| Number of Protons | 17 |
| Number of Neutrons | 18 |
| Number of Electrons | 17 |
How many protons neutrons and electrons are in chlorine 37?
4 rows · Jan 31, 2020 · So in chlorine, we can see that the atomic number is 17. This means that each chlorine atom has ...
How many protons neutrons and electrons are in 35Cl?
How many protons, neutrons, and electrons are there in an atom of Chlorine-39? longitadinal displacement of amas element in a medium aasound wave pases rough ita p by m cos (-at). Consider a sound wave of frequency 440 Hz and wavelength 0.75m. If m-12 m, what is the displacement of an element of air located at12mar time0.11 s. This is the best ...
What is the half-life of each isotope of chlorine?
Dec 08, 2020 · Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
What is the difference between chlorine 36 and 37?
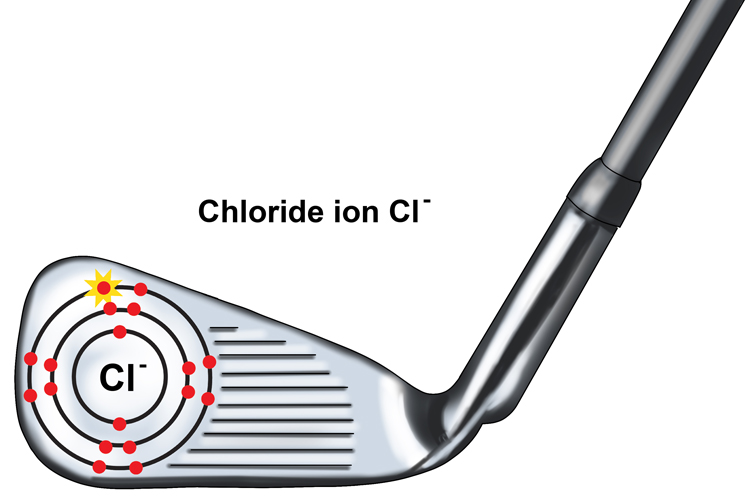
The nucleus of a chlorine atom contains 17 protons and some neutrons. The electron cloud has 17 electrons, so the atom has no overall charge. Chlorine has a tendency to gain one electron. The electron has gone into the space in the electron cloud that is farthest from the nucleus.
How many protons electrons and neutrons are in chlorine 39?
Alright, so this is our final answer, 17 protons, 22 neutrons and 17 electrons.Jan 28, 2022
How many protons does 39 have?
How many neutrons does 39K have? There are 25 known isotopes of potassium, three of which occur naturally: 39K (93.3%), 40K (0.0117%), and 41K (6.7%). Potassium-39 is composed of 19 protons, 20 neutrons, and 19 electrons.Nov 29, 2021
How many protons are in 17n?
Oxygen-17GeneralSymbol17ONamesoxygen-17, O-17Protons (Z)8Neutrons (N)98 more rows
What is the proton number of chlorine 37?
17 protons). Its nucleus contains 17 protons and 20 neutrons for a total of 37 nucleons.
How many neutrons are in chlorine 39?
22List of isotopesNuclideZNExcitation energy39Cl172240Cl172341Cl172435 more rows
How many neutrons does 39 have?
twenty neutronsPotassium-39 has twenty neutrons.Sep 21, 2021
How many protons does chlorine have?
17Chlorine / Atomic number
How many neutrons are in CA?
How many neutrons are in calcium 40?Properties of Calcium-40 Isotope:CALCIUM-40Neutron Number (N)20Atomic Number (Z)20Mass Number (A)40Nucleon Number (A)40Dec 2, 2021
How many neutrons does chlorine have?
18 neutronsEvery chlorine atom has 17 protons in its nucleus. Not all atoms of chlorine have the same number of neutrons, however. Some atoms of chlorine have 18 neutrons in their nuclei. Other atoms of chlorine may have 20 neutrons in the nucleus.
What is the protons of chlorine-35?
17Chlorine-35 / Protons
How many protons does chlorine-35 have?
17 protonsAn element with 17 protons will always be chlorine. However an element's mass numbers can vary, which means that it can have different numbers of neutrons. So although chlorine has a mass number of 35 which means it has 18 neutrons, it can also have a mass number of 37, which means it has 20 neutrons.
Which statement is correct about chlorine-35 and chlorine-37?
Which statement is correct about chlorine-35 and chorine-37? They have the same number of protons and electrons, but a different number of neutrons.
What is the electron configuration of chlorine?
Electron configuration of Chlorine is [Ne] 3s2 3p5. Possible oxidation states are +1,5,7/-1. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
What is the total electrical charge of the nucleus?
The total electrical charge of the nucleus is therefore +Ze , where e (elementary charge) equals to 1,602 x 10-19 coulombs. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.
How many isotopes does chlorine have?
Chlorine has two stable isotopes, 35Cl and 37Cl. These are its only two natural isotopes occurring in quantity, with 35Cl making up 76% of natural chlorine and 37Cl making up the remaining 24%.The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years.
Where are protons and neutrons found?
The protons exist in the nuclei of typical atoms, along with their neutral counterparts, the neutrons. Neutrons and protons, commonly called nucleons, are bound together in the atomic nucleus, where they account for 99.9 percent of the atom’s mass.
Is chlorine a gas?
Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity, behind only oxygen and fluorine.
What is the atomic number?
Since the number of electrons and their arrangement are responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. The configuration of these electrons follows from the principles of quantum mechanics.
How many electrons are in a neutral atom of chlorine?
Therefore, the number of electrons in neutral atom of Chlorine is 17. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.