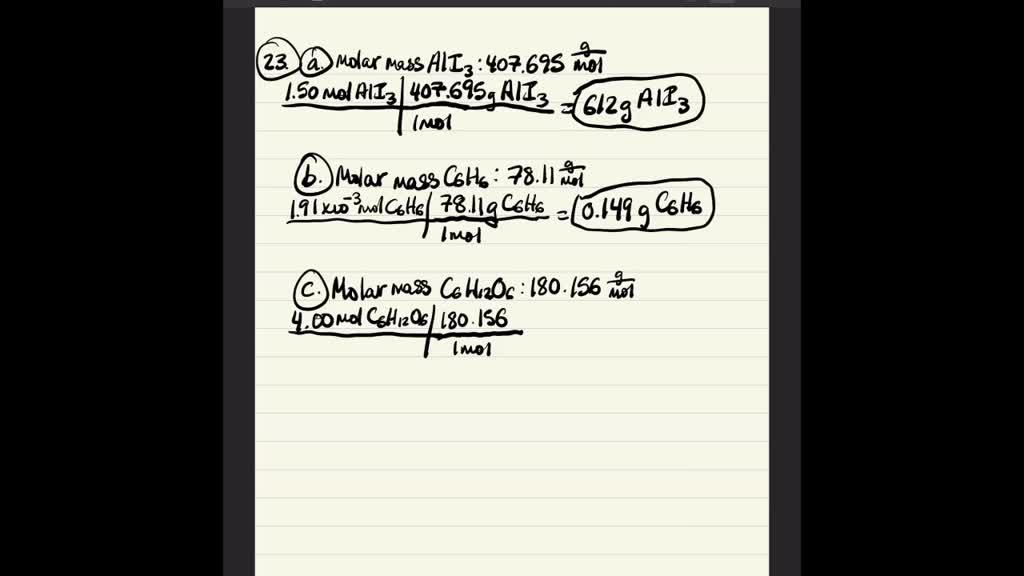
How do you calculate grams from moles?
- A pencil and paper. Calculations are easier to solve when you write them out. Be sure to show all your steps to get full credit.
- A periodic table. You will need to be able to find atomic weight of elements using the periodic table.
- A calculator. Calculators are necessary to simplify calculations of complex numbers.
How to convert grams to moles and vice versa?
Key Takeaways: Converting Moles to Grams (and Vice Versa)
- Grams and moles are two units to express the amount of matter in a sample. ...
- To do this, look up atomic masses on the periodic table and use the formula mass to know how many atoms of each element are in a compound.
- Remember, subscripts in a formula indicate number of atoms. ...
- Multiply the number of atoms of an element by its atomic mass. ...
How many moles are in 1 gram?
›› Quick conversion chart of moles 1 to grams. 1 moles 1 to grams = 1 grams. 5 moles 1 to grams = 5 grams. 10 moles 1 to grams = 10 grams. 20 moles 1 to grams = 20 grams. 30 moles 1 to grams = 30 grams. 40 moles 1 to grams = 40 grams. 50 moles 1 to grams = 50 grams. 75 moles 1 to grams = 75 grams. 100 moles 1 to grams = 100 grams ››
How to find grams per mole?
- H = 1.01 g/mol
- O = 16.00 g/mol
- H 2 O = 2 + 16 = 18 g/mol (look at the subscript to note there are 2 hydrogen atoms)
How many moles is a gram of water?
2:124:18grams to moles water example - YouTubeYouTubeStart of suggested clipEnd of suggested clipNow I add these together and I see the molar mass of water is eighteen point zero one that is theMoreNow I add these together and I see the molar mass of water is eighteen point zero one that is the number of grams of water in one mole of water. So that's the molar mass.
How many moles are in 1 grams?
A mole is an abstract number that correlates to 6.02 x 10^23 units of a substance present. It doesn't matter what it is, one mole of it will be 6.02 x 10^23 units. A gram is a scientific measurement of an object's mass.
Is 1 gram the same as 1 mole?
One mole of a substance has the same mass in grams that one atom or molecule has in atomic mass units. The numbers in the periodic table that we identified as the atomic masses of the atoms not only tell us the mass of one atom in u but also tell us the mass of 1 mol of atoms in grams.
How many moles are in one water?
Water (H2O) is made from 2 atoms of hydrogen and 1 atom of oxygen. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. From the periodic table we see the atomic weight of hydrogen is 1.0079 and the atomic weight of oxygen is 15.9994.
How do I convert grams to moles?
0:5213:17How To Convert Grams To Moles - VERY EASY! - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo here we need to divide. It's going to be 30 divided by 12 which is 2.5. So 30 grams of carbon isMoreSo here we need to divide. It's going to be 30 divided by 12 which is 2.5. So 30 grams of carbon is equivalent to 2.5 moles of carbon.
How do I find moles from grams?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.
How many moles is 25 grams of water?
1.38 molesAs in the given question we have to calculate how many moles are present in the 25 grams of water and for this we have to divide the given weight of water by the molecular weight / mass of water. Hence, 1.38 moles (mol) are present in 25 grams of water.
How many moles are in 50 grams of H2O?
1 Answer. There are 3 moles of O in 50 g.
How many moles are in 36.0 g of H2O?
two molesHence, the number of moles in $36g$ of water is two moles.
How many moles are in 1 ml of water?
We can thus say that for 1 ml of water the number of moles is $\dfrac{1}{{18}}$. So, now we will use the unitary method to calculate the number of molecules present in $\dfrac{1}{{18}}$ moles of water. So, we can conclude that in 1 ml of water the number of molecules present is $3.346 \times {10^{22}}$.
What does 1 mole of H2O weigh?
18.01528 g/molWater / Molar mass
How do I calculate moles?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
How to find the formula weight?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
What is the molecular formula for water?
You can view more details on each measurement unit: molecular weight of Water or grams. The molecular formula for Water is H2O. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Water, or 18.01528 grams. Note that rounding errors may occur, so always check the results.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
How to find the formula weight?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
How much does a mole of water weigh?
Therefore, one mole of water weighs 18.0152 grams. Unless you have a good sense of mass, this value probably doesn't have much meaning to you. It's easier to grasp how much water is in a mole if you find the volume of this amount of mass. Fortunately, this is another simple calculation.
How to find the mass of a mole of water?
Mass of 1 Mole of Water 1 Water (H 2 O) is made from 2 atoms of hydrogen and 1 atom of oxygen. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. 2 From the periodic table we see the atomic weight of hydrogen is 1.0079 and the atomic weight of oxygen is 15.9994. 3 Atomic mass is the number of grams per mole of the element. This means 1 mole of hydrogen weighs 1.0079 grams and 1 mole of oxygen weighs 15.9994 grams.
How many atoms are in water?
Water (H 2 O) is made from 2 atoms of hydrogen and 1 atom of oxygen. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. From the periodic table we see the atomic weight of hydrogen is 1.0079 and the atomic weight of oxygen is 15.9994.
How to find volume of water in a mole?
To find out the volume of water in one mole, you need to know the density of water. The density of water varies depending on temperature and pressure but can usually be taken as 1 gram per milliliter. Density is the amount of mass per unit volume or: Density = Mass/Volume.
What is a mole?
A mole is a unit of measuring the quantity of anything. It is simple to calculate the weight and volume of a mole of water.
How many moles are in grams?
A mass in grams numerically equal to the molecular weight contains one mole of molecules, which is known to be 6.02 x 10^23 (Avogadro’s number). So if you have x grams of a substance, and the molecular weight is y, then the number of moles n = x/y and the number of molecules = n multiplied by Avogadro’s number.
How many mol of water are in 18 g?
Continue Reading. The molar mass of the water molecule is 18 g/mol. It implies that 1 mol of water molecules weigh 18 g. Now you can calculate easily the number of water molecules present in 1 g of water. Calculation follows. 18 g of water contains 1 mol of water molecules.
How many water molecules are in 18g?
18 g of water contains 1 mol of water molecules. or 18 g of water contains 6.022 x 10^23 water molecules. or 1 g of water contains (6.022 x 10^23)/ 18 water molecules and the number comes out to be 3.3455 x 10^22. So the number of water molecules present in 1 g of water is 3.3455 x 10^22. I hope you got the answer to your question.