How many moles are in 25 grams of argon?
In this way, how many moles are in Argon? How many moles are in 25 grams of HF? Using this equation, we can take 25/ (1.0 + 19) and find that it is equal to 1.25 moles.
What is the molecular weight of argon in grams?
The answer is 39.948. We assume you are converting between grams Argon and mole. You can view more details on each measurement unit: molecular weight of Argon or mol The molecular formula for Argon is Ar. Click to see full answer.
How many moles of AR are in 22/40 AR?
Argon mass number is 40. You can approximate it to the whole number while doing the calculations. 22/40 = 0.55 moles. The number of moles of Ar is 0.55 moles. Be carefully when the calculations are related to noble gases.
What is the mass of 1 mole of oxygen in grams?
Oxygen gas is diatomic (meaning two atoms of 16 g/mol each), so 1 mole of O2 has a mass of 32 grams. Divide the amount you have by the molar weight to obtain the number of moles in the sample:
See more
How many moles are in Argon?
To solve this let us find out the molar mass of Argon, it is 39.948 g/mol or roughly 40 g /mol. It means that one mole of Argon weighs 40g. 607g / 40g moles or 15.175 moles.
How many moles are in 22g of Argon AR Brainly?
number of moles of Ar = 22 g / 40 g/mol = 0.55 mol .
How many moles are in 22 grams of carbon?
So, in 22g of CO2 contains 0.5 moles.
What is the mole of 22 gram CO2?
0.5 moleThus, 22 grams of carbon dioxide are equal to 0.5 mole.
How do I calculate moles?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How do you calculate number of moles?
3:095:16GCSE Science Revision Chemistry "Calculating Moles of an Element"YouTubeStart of suggested clipEnd of suggested clipTo work out the number of moles we divide the mass of the element that were given by the relativeMoreTo work out the number of moles we divide the mass of the element that were given by the relative atomic mass.
How many molecules are there in 22 grams of CO2?
= 3.011 × 10²³ molecules.
How do you convert from grams to moles?
0:5213:17How To Convert Grams To Moles - VERY EASY! - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo here we need to divide. It's going to be 30 divided by 12 which is 2.5. So 30 grams of carbon isMoreSo here we need to divide. It's going to be 30 divided by 12 which is 2.5. So 30 grams of carbon is equivalent to 2.5 moles of carbon.
How many atoms are present in 22g of CO2?
Number of carbon atoms present in 22g CO2 is. A. 6.02×1023.
How many moles is 20 grams of CO2?
0.45 molesThe correct answer is 0.45 moles.
What is not correct 22 grams of CO2?
1 CO2 contains 2 atoms of oxygen , hence 1 mole CO2 contains 2 moles of oxygen and therefore moles of oxygen atoms or g-atoms of oxygen are 0.5x2 = 1 g-atom or 1 mole. Hence the incorrect option is (2).
How many moles are in 16g of CO2?
0.5 molesSo 16 g of O2 have 22 g of CO2 or 0.5 moles of it. Very simple as one mole is equal to twelve items so one mole CO2 is 44g here out of which 32g is O2 means if oxygen is 16g then CO2 must be 22g so it is clear that CO2 is 22g as we have to convert it into moles it is 12/22 =0.5 moles which is the answer.
How many moles of Argon Ar are in 452.0 grams?
1 Answer. The number of moles in 452 grams of Argon is 13.31 moles.
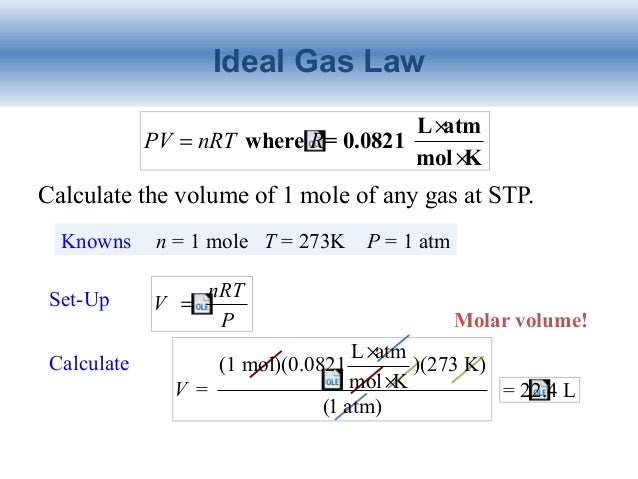
How many moles of Argon atoms are present in 11.2 L of Argon gas at STP?
Using the older standard, as indicated in the explanation, 11.2 L of Ar atoms contain 0.500 mol .
How many moles are in 28 grams of co2?
0.636 molesThe molecular weight of a material is required to tell an individual how many grams there are in one mole of that chemical substance i.e. molecular weight of any substance contains 1 mole of that substance. Then 28 g of $C{{O}_{2}}$ contains = 0.636 moles of $C{{O}_{2}}$.
How many moles of magnesium is 3.01 x10 22 atoms?
0.05 molesMixed Mole ConversionsQuestionAnswerHow many moles of magnesium is 3.01 x 10^22 atoms of magnesium?0.05 molesHow many molecules are there in 4.00 moles of glucose, C6H12O6?2.408 x 10^24Find the mass in grams of 2.00 x 10^23 molecules of C10H15O (called penguinone because its structure looks like a penguin.)50.17 g5 more rows
What is the mass number of argon?
Argon mass number is 40. You can approximate it to the whole number while doing the calculations.
How many grams are in a mole?
One mole of a substance is the amount of grams that is numerically equal to the atomic weight or molecular weight (expressed in atomic mass units, amu). Example: The molecular weight of water is 18 amu, so one mole of water has a mass of 18 grams; two moles have a mass of 2 (18 grams) = 36 grams.
How many moles of iron are in a mole of Fe2O3?
In one mole of fe2o3 there are two moles of iron atoms. Now gram atomic wt.of Fe is 56.So in one mole of Fe2O3 there are 2×56=112 gm of Fe. Hence in 1.75moles Fe2O3 there are 1.75×2 moles or 1.75×56 gm of iron. 11.8K views.
What is mole ratio?
The mole ratio had the form (coefficient of wanted substance/ coefficient of given substance) Convert the moles of the wanted substance to the desired units . In this problem the desired substance is a gas. So you will need to use the ideal gas law to convert from moles to any other unit.
How many electrons does an argon atom have?
An argon atom contains 18 electrons. But if you want to know about the number of electrons in a certain amount of argon , suppose 5 litres of argon gas, then you have to use the avogadro theory.
How to solve stoichiometry problems?
There are four steps to every stoichiometry problem. Write a balanced chemical equation for the reaction. Here it's a decomposition of potassium chlorate into potassium potassium chloride and oxygen gas, O2. Convert the given amount of substance to moles of that substance.
What is the mass of 2.4 moles?
My periodic table of the elements gives the Gram Atomic Mass of natural sulphur is 32.066 grams/mole. Therefore, 2.4 moles would have a mass of 76.96 gm