Full Answer
How many molecules of ammonia NH3 are produced?
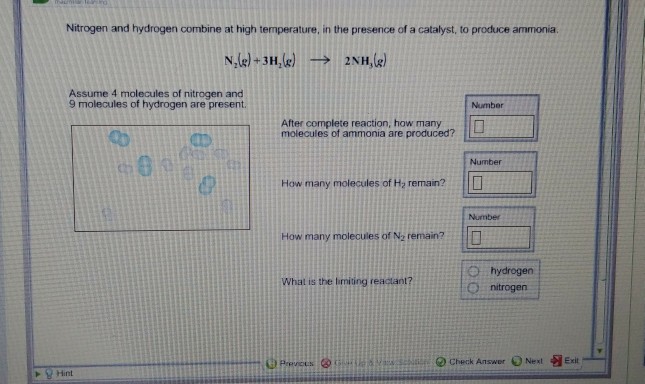
Click to see full answer. Just so, how many molecules of ammonia nh3 are produced? Make the calculations: n (N2)reacted = 1/3*n (H2) = 3 molecules n (NH3)produced = 2/3*n (H2) = 6 molecules The number of unreacted molecules of N2 is 4 – 3 = 1 molecule.
How many molecules of NH3 are in a mole?
how many molecules are there in nh3? 1 Expert Answer molecules NH3 = 1.47 moles x 6.02x1023 molecules/mole = 8.9x1023 molecules of NH3 (to 2 sig. Also asked, when the reaction is complete how many molecules of nh3 are produced?
What is the chemical formula for ammonia?
?) Ammonia is a compound of nitrogen and hydrogen with the formula NH 3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell.
How many molecules of ammonia are produced when nitrogen reacts with hydrogen?
The balanced chemical equation for the reaction between nitrogen and hydrogen to produce ammonia is : N2 + 3H2 ------> 2 (NH3). From the above equation, it can be seen that the ratio of ammonia produced to hydrogen reacted is 2:3. So, the number of ammonia molecules produced = 4*2/3 = 8/3 moles.
See more
How many molecules are in make ammonia?
We see that 1 molecule of nitrogen reacts with 3 molecules of hydrogen to form 2 molecules of ammonia.
What is the molecule name for 2NH3?
Ammonia is a compound of nitrogen and hydrogen with the formula NH 3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell....Ammonia.NamesChemical formulaNH 3Molar mass17.031 g/molAppearanceColourless gasOdorstrong pungent odour73 more rows
How many of each atom do you need to make 2NH3?
2NH3. Question 17 options: 6 nitrogen and 6 hydrogen. 2 nitrogen and 3 hydrogen.
What is N2 3H2 → 2NH3?
For example, nitrogen gas and hydrogen gas react to form. ammonia: N2(g) + 3H2(g) → 2NH3(g) This reaction requires pressures between 2100 and 3600 psi and temperatures. between 300 and 550 oC.
What does the 2 stand for in 2nh3?
Chemical name of is two molecules of nitrogen trihydride.
What is the formula a molecular mass of 2nh3?
17.03052 g/mol .
How many molecules of NH3 are produced from N2 3H2 → 2NH3?
6 moles of diatomic hydrogen X (moles of ammonia/ moles of diatomic hydrogen) = our answer. Therefore, 6 x (2/3) = 4 moles of ammonia.
Is N2 3H2 → 2NH3 balanced?
0:021:00How to Balance: N2 + H2 = NH3 (Synthesis of Ammonia) - YouTubeYouTubeStart of suggested clipEnd of suggested clipAre equal and this equation is balanced.MoreAre equal and this equation is balanced.
How many atoms are there in two molecules of ammonia?
1 Answer. There are four atoms in a molecule of ammonia.
What is the mole ratio of N2 3H2 2NH3?
N2(g) +3H2(g) 2NH3(g) For the reaction initially the mole ratio was 1 : 3 of N2 : H2.
What are the products in N2 3H2 2NH3?
The reactants are Nitrogen {N2} and Hydrogen {H2}. The product is Ammonia {NH3}. Hope this helps!
What is the limiting reactant of N2 3H2 --> 2NH3?
1 Expert Answer 3 mol H2 * (2 mol NH3 / 3 mol H2) = 2 mol NH3. So H2 is the limiting reactant.
How many molecules of NH3 react with 5 molecules of O2?
The reaction above can mean: 4 molecules of NH3 reacts with 5 molecules of O2 to produce 4 molecules of NO and 6 molecules of H2O.
How many moles are in NH3?
Also, how many molecules are there in nh3? 1 Expert Answer molecules NH3 = 1.47 moles x 6.02x1023 molecules/mole = 8.9x1023 molecules of NH3 (to 2 sig.
What is the balance of the chemical equation for the reaction between nitrogen and hydrogen to produce ammonia?
The balanced chemical equation for the reaction between nitrogen and hydrogen to produce ammonia is : N2 + 3H2 ------> 2 (NH3). From the above equation, it can be seen that the ratio of ammonia produced to hydrogen reacted is 2:3. So, the number of ammonia molecules produced = 4*2/3 = 8/3 moles.
How many molecules of nitrogen react with hydrogen?
For example, 10 molecules of nitrogen would react with 30 molecules of hydrogen to produce 20 molecules of ammonia.
What is the chemical name for ammonia?
Chemical compound. Ammonia is a compound of nitrogen and hydrogen with the formula NH 3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct characteristic of a pungent smell.
How much ammonia is in water?
Industrial ammonia is sold either as ammonia liquor (usually 28% ammonia in water) or as pressurized or refrigerated anhydrous liquid ammonia transported in tank cars or cylinders. NH 3 boils at −33.34 °C (−28.012 °F) at a pressure of one atmosphere, so the liquid must be stored under pressure or at low temperature.
How long can you be exposed to ammonia?
The U.S. Occupational Safety and Health Administration (OSHA) has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume. The National Institute for Occupational Safety and Health (NIOSH) recently reduced the IDLH (Immediately Dangerous to Life and Health, the level to which a healthy worker can be exposed for 30 minutes without suffering irreversible health effects) from 500 to 300 based on recent more conservative interpretations of original research in 1943. Other organizations have varying exposure levels. U.S. Navy Standards [U.S. Bureau of Ships 1962] maximum allowable concentrations (MACs): continuous exposure (60 days): 25 ppm / 1 hour: 400 ppm. Ammonia vapour has a sharp, irritating, pungent odour that acts as a warning of potentially dangerous exposure. The average odour threshold is 5 ppm, well below any danger or damage. Exposure to very high concentrations of gaseous ammonia can result in lung damage and death. Ammonia is regulated in the United States as a non-flammable gas, but it meets the definition of a material that is toxic by inhalation and requires a hazardous safety permit when transported in quantities greater than 13,248 L (3,500 gallons).
What are the characteristics of ammonia?
One of the most characteristic properties of ammonia is its basicity. Ammonia is considered to be a weak base. It combines with acids to form salts; thus with hydrochloric acid it forms ammonium chloride (sal ammoniac); with nitric acid, ammonium nitrate, etc. Perfectly dry ammonia gas will not combine with perfectly dry hydrogen chloride gas; moisture is necessary to bring about the reaction.
How much ammonia is used in fertilizer?
In the US as of 2019, approximately 88% of ammonia was used as fertilizers either as its salts, solutions or anhydrously. When applied to soil, it helps provide increased yields of crops such as maize and wheat. 30% of agricultural nitrogen applied in the US is in the form of anhydrous ammonia and worldwide 110 million tonnes are applied each year.
What is the process of decomposition of ammonia?
Decomposition of ammonia is a slightly endothermic process requiring 23 kJ/mol (5.5 kcal/mol) of ammonia, and yields hydrogen and nitrogen gas.
How to detect ammonia?
Ammonia and ammonium salts can be readily detected, in very minute traces, by the addition of Nessler's solution, which gives a distinct yellow colouration in the presence of the slightest trace of ammonia or ammonium salts. The amount of ammonia in ammonium salts can be estimated quantitatively by distillation of the salts with sodium or potassium hydroxide, the ammonia evolved being absorbed in a known volume of standard sulfuric acid and the excess of acid then determined volumetrically; or the ammonia may be absorbed in hydrochloric acid and the ammonium chloride so formed precipitated as ammonium hexachloroplatinate, (NH 4) 2 PtCl 6.
How many moles of ammonia must be produced?
6 * 2/3 moles of ammonia must be produced.
How many molecules does N2 react with?
2molecules N2 react with 6 molecules H2 to produce 4 molecules NH3 and there will be 4 molecules H2 unreacted.
Why does hydrogen run out before nitrogen?
Hydrogen is the limiting reactant. In other words, Hydrogen will run out before Nitrogen because it takes 3 mole of H2 and just 1 mole of N2 to make 2 mole NH3.
What is the ratio of nitrogen to hydrogen?
The equation for the reaction of nitrogen and hydrogen to give ammonia is. N2 + 3 H2 → 2 NH3. The ratio of N2/H2 is 1:3 but the question says the ratio is 4/8 or 1/2. This means nitrogen is in excess and when you are done consuming all of the hydrogen, you will have some nitrogen left.
What is the number of a mole of hydrogen?
Avogadro’s number is the conversion factor from atomic mass units (AMU) to grams (1 gram = 6.02 * 10^23 AMU). Thus one mole of hydrogen has a mass of 1 gram.
How many atomic masses can you calculate without using the atomic mass?
So the answer is four . You can do this sort of calculation without using the atomic masses
How to divide molar mass?
Since molar mass is a fraction (g/mol), you can divide by multiplying by its reciprocal (mol/g). This makes it easier to see how grams cancel, leaving moles.