Which elements are in period 4 of the periodic table?
Today, 150 years later, chemists officially recognize 118 elements (after the addition of four newcomers in 2016) and still use Mendeleev's periodic ... Load Error The table has seven rows and 18 columns. Each row represents one period; the period number ...
What alkali metal is in fourth period of periodic table?
They are: lithium (Li) sodium (Na) potassium (K) rubidium (Rb) caesium (Cs) francium (Fr) Properties of alkali metals: The alkali metals have the high thermal and electrical conductivity, lustrous, ductile, and malleable that are characteristic of metals.
What are the 4 categories of the periodic table?
- Lithium (Li)
- Sodium (Na)
- Potassium (K)
- Rubidium (Rb)
- Cesium (Cs)
- Francium (Fr)
What is the fourth period?
The period 4 transition metals are scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn). How many electrons are there in the fourth period? 2 Answers. BRIAN M. The fourth energy level has 18 electrons.
How many elements does fourth and fifth period of modern periodic table contain?
18 elementsThe correct answer is 18 elements. The 4th and 5th-period contains 18 elements each.
How many elements can be accommodated in the 4th period of the modern periodic table?
18 elementsTherefore, 9 orbitals, at the maximum, can have 18 electrons and hence, fourth period has 18 elements.
Which elements belong to the 4th period?
Bromine is the only element present in period 4 while other elements belong to different periods.
How many elements are present in the 4th and 6th periods in the modern periodic table?
Period 1 has only two elements (hydrogen and helium), while periods 2 and 3 have 8 elements. Periods 4 and 5 have 18 elements. Periods 6 and 7 have 32 elements because the two bottom rows that are separated from the rest of the table belong to those periods.
How many orbitals are there in 4th period?
Fourth period means the principal quantum number is 4 i.e there are 4 shells in the elements of the 4th period. and f sub shell contains 7 orbitals. So total number of orbitals in elements of 4th period is 30.Sep 30, 2017
How many elements can be accommodated in each period?
The number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled.Dec 28, 2013
How many elements are placed in 3rd and 4th period of modern periodic table?
The total number of elements present in the 4th period of the modern periodic table is 18. These include two s block elements, 6 p block elements and 10 d block elements. Was this answer helpful?
How elements are arranged in 4th period?
Arrangements of elements in period of the periodic table. The reason for the arrangement of elements in the period is based upon the atomic number. In all periods, including the elements are arranged according to the atomic number in increasing order.Dec 22, 2016
What element is in group 5 period 4?
ZrGroup 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi)....Group 5A — The Pnictogens.ZrNbMoTc4ASn12 more columns
How many elements are present in 4th 5th 6th and 7th period?
There are 2 elements in the first period, 8 elements in second and third periods each respectively, 18 elements fourth and fifth periods each respectively, 32 elements in the sixth period, and 19 elements in the seventh period till now.
How many electrons are present in 4th period?
18 electronsBRIAN M. The fourth energy level has 18 electrons. The fourth energy level of the periodic table includes the 4s 3d and 4p orbitals.May 10, 2014
How many elements are present in the third fourth and sixth periods of the periodic table?
The 1st period consists of only two elements – Hydrogen and Helium. While the 2nd and 3rd period consists of 8 elements each. On the other hand, the 6th period consists of 32 elements.Aug 20, 2020
How many columns are there in the periodic table?
There are eighteen vertical columns known as groups in the modern periodic table which are arranged from left to right and seven horizontal rows which are known as periods. The elements of this group form salts. They are noble gases and under normal conditions they are inert.
What is the long group of elements?
Period four and five have eighteen elements and are known as the long group. In the modern periodic table, group number 3 of period six contains the lanthanide series which are the rare earth elements. We have radioactive elements (actinides) present in group 3 of period seven.
Why do atomic radii decrease?
The atomic radii of the elements generally decrease across periods due to the increase in electronegativity and the increase in the effective nuclear charge acting on the outermost shells.
How many elements are in a vertical column?
Earlier scientists assumed that the properties of elements are periodic functions of their atomic masses. On the basis of this assumption, Mendeleev placed 63 elements in a vertical column called groups and in horizontal rows called periods.
Which element has the lowest electronegativity?
In the modern periodic table, the electronegativity of elements increases across a period (row) and decreases down a group (column). Therefore, the bottom-left most element (francium) is predicted to have the lowest electronegativity and the top-right most element (fluorine) is predicted to have the highest electronegativity.
What are the elements of group 18?
The elements of group 1, 2, 13, 14, 15, 16, and 17 are known as the main group elements or normal elements. The elements of groups 3, 4, 5, 6, 7, 8, 9, 11 and 12 are known as the transition elements. Group 18 is called noble gases or inert gases. Their outermost shell is completely filled. Due to this stable electronic configuration, they generally don’t react with the other elements.
Which group of metals form strong alkalis with water?
Alkali metals. They form strong alkalis with water. Group 2 or IIA. Alkaline earth metals. They also form alkalis but weaker than group 1 elements. Group 13 or IIIA. Boron family. Boron is the first member of this family. Group 14 or IVA.
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
How many categories are there in the periodic table?
The elements of the periodic table shown here are divided into nine categories; six for the metals, and two for nonmetals, and a metalloid category. The nine categories (or sets) correspond to those found in the literature for the applicable part of the periodic table. Different authors may use different categorisation schema depending on the properties of interest.
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What is the electron configuration of a neutral atom?
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered 1, 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram. The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
How many electrons are in neon?
The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to ...
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
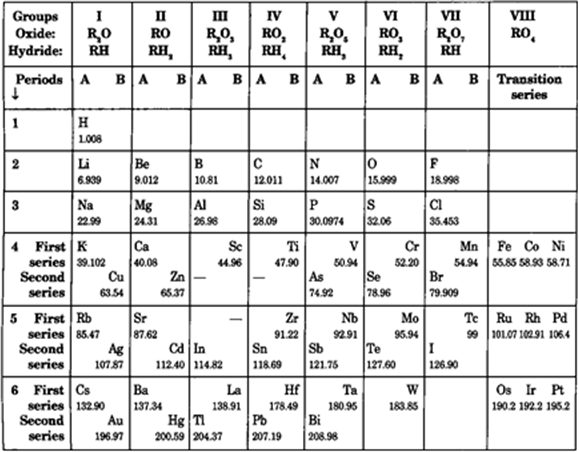
Modern Periodic Table
Bohr is credited for developing the current periodic table. It is also known as the periodic table in its extended version. In the contemporary table, the elements are grouped in horizontal rows called periods in increasing atomic number order.
What are Periods?
In a periodic table, periods are the horizontal rows of elements. There are seven periods in the long form of the periodic table. The elements in the periodic table have consecutive atomic numbers. Each period has a different number of elements, as shown below.
To decide whether an element comes first or last in a period?
The first period begins with hydrogen and finishes with a noble gas, helium. All other periods begin with alkali metals such as lithium, sodium, and potassium and end with noble gases such as neon, argon, and krypton.
What are Groups?
In a periodic table, groups are the vertical columns. In the long form of the periodic table, there are 18 groups. These groups are numbered from one to eighteen. In a particular group, the elements do not have consecutive atomic numbers. On the left side of the periodic table, is group 1 whereas on the right side of the periodic is group 18.
Metals and Non-metals in the Modern Periodic Table
The elements have been roughly divided into metals and non-metals, in the modern periodic table. The elements on the left side of the table are metals whereas the elements on the right side of the table are non-metals.
Position of Hydrogen in the Modern Periodic Table
Since hydrogen has an electronic configuration that is similar to that of alkali metals, it has been placed at the top of group 1 above them. Both hydrogen and alkali metals have a single valence electron. Since the hydrogen atom is so small, many of its properties differ from those of alkali metals.
Solved Questions
Question 1: Give some examples of elements with only one electron in their outermost shells.
Overview
A period 4 element is one of the chemical elements in the fourth row (or period) of the periodic table of the elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The fourth period contains 18 elements beginning with potassium and e…
Properties
Every single one of these elements is stable, and many are extremely common in the Earth's crust and/or core; it is the last period with no unstable elements at all. Many of the transition metals in period 4 are very strong, and therefore commonly used in industry, especially iron. Three adjacent elements are known to be toxic, with arsenic one of the most well-known poisons, selenium being toxic to humans in large quantities, and bromine, a toxic liquid. Many elements are essential to h…
List of elements
Chemical element Block Electron configuration 19 K Potassium s-block [Ar] 4s 20 Ca Calcium s-block [Ar] 4s 21 Sc Scandium d-block [Ar] 3d 4s 22 Ti Titanium d-block [Ar] 3d 4s 23 V Vanadium d-block [Ar] 3d 4s 24 Cr Chromium d-block [Ar] 3d 4s (*) 25 Mn Manganese d-block [Ar] 3d 4s 26 Fe Iron d-block [Ar] 3d 4s 27 Co Cobalt d-block [Ar] 3d 4s 28 Ni Nickel d-block [Ar] 3d 4s 29 Cu Copper d-block [Ar] 3d 4s (*) 30 Zn Zinc d-block [Ar] 3d 4s 31 Ga Gallium p-block [Ar] 3d 4s 4p 3…
Chemical element Block Electron configuration 19 K Potassium s-block [Ar] 4s 20 Ca Calcium s-block [Ar] 4s 21 Sc Scandium d-block [Ar] 3d 4s 22 Ti Titanium d-block [Ar] 3d 4s 23 V Vanadium d-block [Ar] 3d 4s 24 Cr Chromium d-block [Ar] 3d 4s (*) 25 Mn Manganese d-block [Ar] 3d 4s 26 Fe Iron d-block [Ar] 3d 4s 27 Co Cobalt d-block [Ar] 3d 4s 28 Ni Nickel d-block [Ar] 3d 4s 29 Cu Copper d-block [Ar] 3d 4s (*) 30 Zn Zinc d-block [Ar] 3d 4s 31 Ga Gallium p-block [Ar] 3d 4s 4p 3…
s-block elements
Potassium (K) is an alkali metal, placed under sodium and over rubidium, and is the first element of period 4. It is one of the most reactive elements in the periodic table, therefore usually only found in compounds. It tends to oxidize in air very rapidly, thus accounting for its rapid reaction with oxygen when freshly exposed to air. When freshly exposed, it is rather silvery, but it quickly begins to tarnish as it reacts with air. It is soft enough to be cut with a knife and it is the second least de…
d-block elements
Scandium (Sc) is the third element in the period, and is the first transition metal in the periodic table. Scandium is quite common in nature, but difficult to isolate because it is most prevalent in rare earth compounds, which are difficult to isolate elements from. Scandium has very few commercial applications because of the aforementioned facts, and currently its only major application is in aluminium alloys.
p-block elements
Gallium (Ga) is an element in group 13, under aluminium. Gallium is noteworthy because it has a melting point at about 303 kelvins, right around room temperature. For example, it will be solid on a typical spring day, but will be liquid on a hot summer day. Gallium is an important component in the alloy galinstan, along with tin. Gallium can also be found in semiconductors.
Germanium (Ge) is an element in group 14. Germanium, like silicon above it, is an important semic…
Biological role
Many period 4 elements find roles in controlling protein function as secondary messengers, structural components, or enzyme cofactors. A gradient of potassium is used by cells to maintain a membrane potential which enables neurotransmitter firing and facilitated diffusion among other processes. Calcium is a common signaling molecule for proteins such as calmodulin and plays a critical role in triggering skeletal muscle contraction in vertebrates. Selenium is a component of the