What is the chemical equation for hydrogen chloride?
The compound hydrogen chloride has the chemical formula H Cl and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor.
How does hydrogen and chloride react to form hydrogen chloride?
Procedure
- Working in the fume cupboard, clamp the glass tube at either end, ensuring that it is horizontal.
- Open the bottle of ammonia solution cautiously, pointing the bottle away from both you and the audience. ...
- Put one of the cotton wool wads in the mouth of the ammonia bottle and carefully invert it to soak one side of it. ...
Is hydrogen chloride same as hydrochloric acid?
Hydrogen chloride gas and hydrochloric acid have the same chemical formula: HCl. The difference between the two is that hydrogen chloride is a gas, and hydrochloric acid is an aqueous solution. In HCl (g), the hydrogen atom shares two electrons with the chlorine atom.
What is the Lewis structure for hydrogen chloride?
Steps of drawing lewis structure of HCl
- Total number of electrons of the valance shells of HCl. There are two elements in hydrogen chloride; hydrogen and chlorine. ...
- Total valence electrons pairs. ...
- Center atom of HCl molecule. ...
- Lone pairs on atoms. ...
- Mark charges on atoms. ...
- Check the stability and minimize charges on atoms by converting lone pairs to bonds. ...
How do you write the formula of hydrogen chloride in Class 9?
C: HCl2.
How do you write the name HCl?
0:333:38How to name HCl - Real Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd the base name of chlorine is chlor. And so we're just going to write chlor. And now we go aheadMoreAnd the base name of chlorine is chlor. And so we're just going to write chlor. And now we go ahead and we add ick we always add ick to the base name of our second element. So it becomes hydrochloric.
How HCl is formed?
Preparation of Hydrogen Chloride Muriatic acid is prepared by warming NaCl crystals with concentrated H2SO4 (Sulphuric acid). Usually, most of the hydrogen chloride/hydrochloric acid that is formed is a co-product of some other chemical reactions. HCl is also formed by the chlorination of hydrocarbons.
How do you write hydrogen formula?
Hydrogen has a molar mass of 1 and it's molecular formula is H2.
How do you write acid formulas?
Naming and Formulas for AcidsAs a general rule the formula for an acid starts with hydrogen.If acid consists of just two elements, then it is named Hydro-_______-ic Acid.For example HCl is Hydrochloric Acid.And the formula for Hydrobromic Acid is HBr.
What does HCl stand for?
Hydrochloric acid is an aqueous (water-based) solution of the gas, hydrogen chloride. Hydrochloric acid is an aqueous (water-based) solution of the gas, hydrogen chloride.
What is HCl made of?
Hydrochloric acid (HCl) is a corrosive and strongly acidic solution that is made by dissolving gaseous hydrogen chloride in water. It is made industrially as well as in the body.
What does HCl look like?
At room temperature, hydrogen chloride is a colorless to slightly yellow, corrosive, nonflammable gas that is heavier than air and has a strong irritating odor. On exposure to air, hydrogen chloride forms dense white corrosive vapors.
How is hydrogen chloride prepared from NaCl?
We produce Hydrogen Chloride in the laboratory by treating sodium chloride with concentrated sulphuric acid. We, then, heat this mixture up to 420K. We get Sodium bisulphate as a by-product which is insoluble. Therefore, we further mix it with more sodium chloride.
Why do we write hydrogen as H2?
Because there are two hydrogen atoms, we call this diatomic hydrogen, di meaning two. Because the hydrogen atoms are covalently bonded together they form a molecule; so H2 is also referred to as molecular hydrogen. We can also refer to it as dihydrogen.
Is hydrogen H2 or H?
H2 = Molecular Hydrogen H2 is also called molecular hydrogen.It consists of two protons and two electrons. Consequently it is the most common form of Hydrogen because it is stable with a neutral charge.
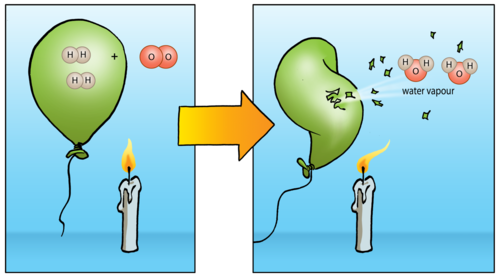
What type of reaction is H2 o2 → h2o?
0:511:11Type of Reaction for H2 + O2 = H2O - YouTubeYouTubeStart of suggested clipEnd of suggested clipThis is dr b with the type of reaction for h2 plus o2 yields h2o hydrogen gas plus oxygen. Gives usMoreThis is dr b with the type of reaction for h2 plus o2 yields h2o hydrogen gas plus oxygen. Gives us water it's both a combination. And a combustion reaction.
How many hydrogens are in the reactants side of the equation?
The equation is balanced as in the reactants side there are two hydrogens present and the same on the products side. For chlorine as well the same thing goes two chlorine atoms on the reactants side and two on the products side.
What is balancing chemical equations?
Answer: Balancing chemical equation refers to balancing the stoichiometric coefficients on the reactants and products side. This must be done as the chemical equation obeys the law of conservation of mass and momentum.