Why is AlCl3 a Lewis acid?
In AlCl3 it forms three bonds and hence outer shell has 6 electrons. Now Al needs two more electrons to complete its octet. By definition those which accepts electrons are called lewis acids. So AlCl3 is a Lewis acid.
What is the Lewis structure of Al3+?
The Lewis structure of water suggests that this molecule has nonbonding pairs of valence electrons and can therefore act as a Lewis base. The electron configuration of the Al 3+ ion suggests that this ion has empty 3 s, 3 p, and 3 d orbitals that can be used to hold pairs of nonbonding electrons donated by neighboring water molecules.
What are Lewis acids and bases?
A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH 3 is a Lewis base, because it can donate its lone pair of electrons.
Is NH3 a Lewis base or Lewis acid?
For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me 3 B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond.
Can Al 3 be a Lewis acid?
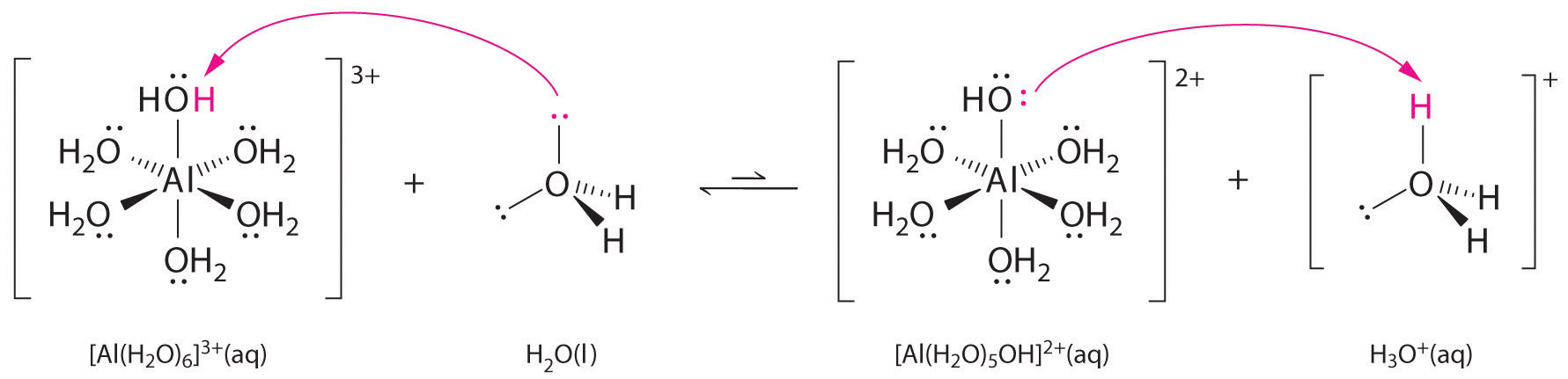
The aluminum ion is the metal and is a cation with an unfilled valence shell, and it is a Lewis Acid. Water has lone-pair electrons and is an anion, thus it is a Lewis Base. Figure 3: Aluminum ion acts as a Lewis acid and accepts the electrons from water, which is acting as a Lewis base.
Is Al 3 +) acidic or basic?
Al3+ is a small ion with high charge, therefore it has very high charge density and high polarizing power. It will grab OH- from water, resulting in more H+ in the solution. The H+ results in the acidic solution.
Is Aluminium chloride a Lewis acid?
Aluminum chloride (AlCl3) is a Lewis acid since an open valence shell is found in the aluminium atom.
Is Al3+ an acid base neutral ion?
Al3+ is an interesting case: Near neutral pH, Al2O3 is insoluble because Al3+ is a very strong Lewis acid. At low pH, the oxide ion is removed by H+, and Al3+ is obtained in solution (as the solvated ion).
Can Al3+ act as a Lewis base?
As an example of a reaction not described by the Bronsted-Lowry definition, Al3+ in water is a Lewis acid. It reacts with water to form an aqua complex: the Al3+ accepts an electron pair from water molecules with the water acting as a Lewis base.
What is the name of Al 3+?
aluminum ionAl+3 is “aluminum ion”.
What is the Lewis structure of AlCl3?
In the AlCl3 lewis structure, a total of 9 lone pairs are present but no lone pair on the central atom. The molecular geometry of AlCl3 is trigonal planar with each Al-Cl bond angeled 120° to each other and its electron geometry is also trigonal planar.
Why are cations Lewis acids?
Cations are electron-deficient species and can accept an electron pair. Hence, cations are Lewis acids.
Which of the following is not a Lewis acid?
The correct answer is Barium chloride (BaCl2). BaCl2, barium chloride is not a Lewis acid because barium loses two electrons while chlorine gains one electron thus forming chloride ion.
What is the electron configuration of Al 3 +?
[Ne] 3s² 3p¹Aluminium / Electron configuration
Is ALCL3 acidic basic or neutral explain with relevant equation?
ALCL3 is acidic in nature.
How is Al3+ produced?
Al3+ is formed by removal of 3 electrons from Al atom. As we know the most stable elements in periodic table are noble gas. They have 8 electrons in their valence shell which is known as octet. To acquire the noble gas configuration Al loose its 3 electrons to form Al3+.
Which is stronger, AlF3 or AlCl3?
AlF3 is the stronger acid between AlF3 and AlCl3. This is because fluorine is more electronegative than chlorine, which means that fluorine has a greater affinity for electrons than chlorine and thus will tend to pull the electrons away from the Al atom.
How many electrons does AlCl3 have?
AlCl3 is electron deficient. It has three electrons in its valence shell. So when it forms a covalent compound with chlorine it forms three single bonds with chlorine. It doesn't form an octet by doing so thus it can take two more electrons by forming a coordinate bond to become an octet and thus it behaves as Lewis acid.
Is AlCl3 an acid?
According to Lewis definition if any compound or ions accept electrons , they are called Lewis acid . Now AlCl3 is an electron deficient compound , it can accept electron from the electron rich molecules or ions due to presence of incomplete octet . So AlCl3 is a Lewis acid . 1.6K views. Sponsored by FinanceBuzz.
Is FeCl3 more acidic than AlCl3?
In comparison between two species , let FeCl3 and AlCl3 ; more is the deficient of electron to complete octate or stable form more is the acidic strength. As AlCl3 have vacant orbital to fulfil octate ,where as FeCl3 have already octate form so AlCl3 is more acidic than FeCl3 .
What are Lewis acids?
Most compounds considered to be Lewis acids require an activation step prior to formation of the adduct with the Lewis base. Well known cases are the aluminium trihalides, which are widely viewed as Lewis acids. Aluminium trihalides, unlike the boron trihalides, do not exist in the form AlX 3, but as aggregates and polymers that must be degraded by the Lewis base. A simpler case is the formation of adducts of borane. Monomeric BH 3 does not exist appreciably, so the adducts of borane are generated by degradation of diborane:
What are the methods used to determine Lewis acidity?
Many are based on spectroscopic signatures such as shifts NMR signals or IR bands e.g. the Gutmann-Beckett method and the Childs method.
What are the classes of Lewis bases?
Typical Lewis bases are conventional amines such as ammonia and alkyl amines. Other common Lewis bases include pyridine and its derivatives. Some of the main classes of Lewis bases are. amines of the formula NH 3−x R x where R = alkyl or aryl.
Which metal ions are coordinatively unsaturated?
Metal ions such as Na +, Mg 2+, and Ce 3+, which are invariably complexed with additional ligands, are often sources of coordinatively unsaturated derivatives that form Lewis adducts upon reaction with a Lewis base. Other reactions might simply be referred to as "acid-catalyzed" reactions.
Is a proton a Lewis acid?
The proton (H + ) is one of the strongest but is also one of the most complicated Lewis acids. It is convention to ignore the fact that a proton is heavily solvated (bound to solvent). With this simplification in mind, acid-base reactions can be viewed as the formation of adducts:
Is Lewis acid a base or acceptor?
Nevertheless, Lewis suggested that an electron-pair donor be classified as a base and an electron-pair acceptor be classified as acid. A more modern definition of a Lewis acid is an atomic or molecular species with a localized empty atomic or molecular orbital of low energy.