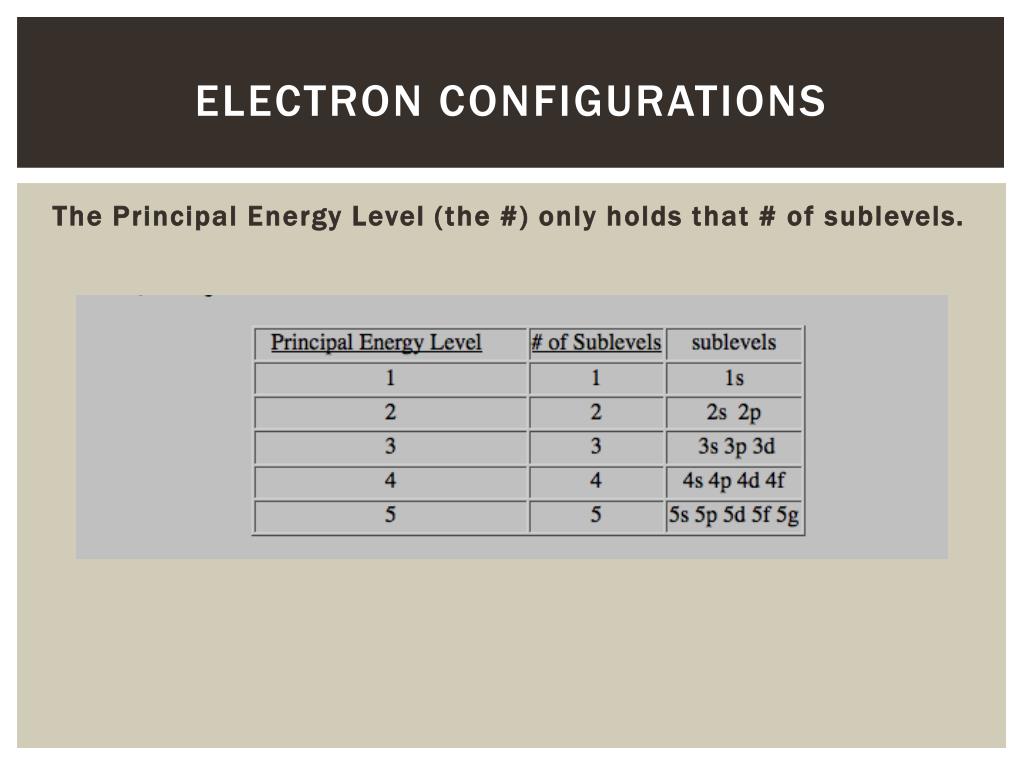
How do you find the principal energy level?
- The atoms in the first period have electrons in 1 energy level.
- The atoms in the second period have electrons in 2 energy levels.
- The atoms in the third period have electrons in 3 energy levels.
- The atoms in the fourth period have electrons in 4 energy levels.
What are the 4 principal energy levels?
What energy level has the highest energy?
- Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found.
- Q: In the atomic model Figure above, where would you find electrons that have the most energy?
- A: Electrons with the most energy would be found in energy level IV.
What is the highest principal energy level?
- These are spherical
- Every energy level contains one S-Orbital
- An S-Orbital in the first energy level is a 1s orbital
- An S-Orbital in the second energy level is a 2s orbital etc.
What is the definition of main energy level?
Define energy level. energy level synonyms, energy level pronunciation, energy level translation, English dictionary definition of energy level. n. 1. The energy characteristic of a stationary state of a physical system, especially a quantum mechanical system. 2. The stationary state of a quantum...
What is the main energy level?
The main energy level that can hold only two electrons. First. For an electron in an atom to change from the ground state to an excited state, Energy must be absorbed. List atomic orbitals in the correct order they are filled according to the Aufbau principle. 1s 2s 2p 3s 3p 4s 3d 4p 5s.
How do you find the energy level of an electron?
1 AnswerE=−13.6n2 where the energy is in electron volts.n is the principle quantum number.So for an electron in n=1 :E=−13.6eV.To convert to joules you can x this by 1.6×10−19.
What are the principles of energy levels?
Electrons are added to atomic orbitals in order from low energy (bottom of graph) to high (top of graph) according to the Aufbau principle. Principal energy levels are color coded, while sublevels are grouped together and each circle represents an orbital capable of holding two electrons.
How many energy levels are there?
Electron Shells, Subshells & Atomic Orbitals The highest energy level number (1 through 7) for the electrons in an atom corresponds to the period (or row) in the periodic table to which that atom belongs. Because there are 7 periods in the table, there are 7 energy levels.
How many principal electron energy levels are there?
seven principal electron energy levelsThere are seven principal electron energy levels.
What is the principal energy level of an electron?
In chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. This level is denoted by the principal quantum number n. The first element in a period of the periodic table introduces a new principal energy level.
How many electrons are in a principal energy level?
A principal energy level may contain up to 2n 2 electrons, with n being the number of each level. The first energy level can contain 2 (1) 2 or two electrons; the second can contain up to 2 (2) 2 or eight electrons; the third can contain up to 2 (3) 2 or 18 electrons, and so on.
How many electrons can a third energy level hold?
The third principal energy level has one s orbital, three p orbitals, and five d orbitals, which can each hold up to 10 electrons. This allows for a maximum of 18 electrons. The fourth and higher levels have an f sublevel in addition to the s, p, and d orbitals.
What is the electron notation?
The notation used to indicate the type of energy level and the number of electrons in that level has a coefficient for the number of the principal energy level, a letter for the sublevel, and a superscript for the number of electrons located in that sublevel. For example, the notation 4p 3 indicates the fourth principal energy ...
How do electrons change energy levels?
The energy of an energy level increases the further out from the nucleus it is. The lower the number of a principal energy level, the closer together the electrons are to each other and to the nucleus of the atom. During chemical reactions, it's more ...
How many electrons can a s orbital hold?
The s orbital can contain a maximum of two electrons. The next principal energy level contains one s orbital and three p orbitals. The set of three p orbitals can hold up to six electrons. Thus, the second principal energy level can hold up to eight electrons, two in the s orbital and six in the p orbital. The third principal energy level has one s ...