Difference between Ketone and Ester
- Definition of Ketone vs. Ester. ...
- Acidity. A ketone is a more acidic molecule. ...
- Reactivity. Ketones have more reactivity while esters have less reactivity.
- Formation. The oxidation of alcohols and hydrocarbons can produce a ketone as can the hydration of alkynes. ...
- Industrial uses. ...
- Examples in humans. ...
What is the difference between a ketone and an ester?
A ketone has a molecular structure that includes a carbonyl bonded to carbons while an ester has a molecular structure in which a carbonyl is bonded to an alkoxy group. What is Ketone? A ketone is a molecule in which a carbon atom is covalently bonded to an oxygen atom to make a carbonyl group and carbons are bonded to this carbonyl.
Do ketone and carboxyl acids have a +I directing group?
However, ketone, carboxyl acid, ester, or amide have an additional +I directing group in R, OH, OR’ and NH2 respectively, which further reduces the In any carbonyl group, the C=O bond is polarized towards O to give a slight +ve charge on the carbonyl carbon.
Why is ester a weaker nucleophile than ketone?
Bookmark this question. Show activity on this post. I guess the ester is a weaker nucleophile because it does have an additional oxygen atom, unlike the ketone, that is pulling electrons from the C-O double bond towards the carbon atom (this happens vice versa too of course with the ester oxygen).
Why are ketones more acidic than bases?
The more stable the base is ,the more acidic the compound is .For ketones we have enolate ion and this enolate ion is resonance stabilised.
Why is an ester more basic than a ketone?
The alkoxy group can stabilize the positive charge by mesomeric effect and the third resonance form makes full sense. That's why esters are more basic than ketones. The fact that, in a protonated carboxylic acid, the first and third resonance forms are equivalent gives an extra of stabilization.
Are esters less acidic than ketones?
In the ester, there is also a resonance donation from the alkoxy group towards the carbonyl that competes with the stabilisation of the enolate charge. This makes the ester enolate less stable than those of aldehydes and ketones so esters are even less acidic.
Are ketones more acidic than ethers?
That means, the hydrogen atom bonded to the carbon atom and is adjacent to the C-O-C bond releases easily in the from of a proton. However, it is less acidic than that of carbonyl compounds such as ketones. Ethers cannot form hydrogen bonds with each other.
Why is alpha hydrogen of ketone more acidic than ester?
The alpha-hydrogen of ketones (pKa = 20) is more acidic as compared to the alpha-hydrogens of esters (pKa = 25). The reason for this is that the ester functional group has free lone pairs on the oxygen which can participate in resonance with carbonyl group.
How is ketone different from ester?
A ketone is a molecule that has a carbonyl bonded to carbons. An ester is a molecule that has a carbonyl and alkoxy group bonded together.
Is an ester or ketone more reactive?
Since the -OR group is a stronger electron donor (resonance) than the alkyl group of the ketone, the ester is less reactive than the ketone... so we get : (b) The aldehyde, carboxylic acid and ester will be reduced to the same product, benzyl alcohol.
What is the difference between ketone and ether?
The main difference between ether and ketone is that the functional group of ether is composed of two carbon atoms bonded to the same oxygen atom whereas the functional group of ketone is composed of an oxygen atom bonded to a carbon atom via a double bond.
Are ketones more acidic than carboxylic acids?
Carboxylic acids generally have pKas in the range of 3 - 5, and therefore are weaker acids than hydronium ion (H3O+), but they are stronger acids than other organic acids, such as alcohols (16 - 20), aldehydes and ketones (18 - 22), alkynes (25), benzene (35) or alkanes (50).
Are ketones acidic?
Ketones are acidic molecules, so an increased level of ketones can cause the blood to become more acidic which prevents the body's processes from working normally.
Is ketone more acidic than alcohol?
Alcohols in general are more acidic than ketones because in aldehydes/ketones, you lose an alpha H to make a carbanion, which is more unstable than a negative charge residing on a more electronegative O in alcohols. First ketones are not acidic.
Are ketones more acidic than alkanes?
It attracts the electron density towards itself and hydrogen ion elimination will be facile. But in alkane no such carbonyl group is present and the resonance of enolate can not be taken place. Thus, the conjugate base of alkane has no stabilization factor. Thus, it shows less acidity than ketone.
Why are esters that contain alpha hydrogen acidic?
The presence of these overlapping p orbitals gives α hydrogens (Hydrogens on carbons adjacent to carbonyls) special properties. In particular, α hydrogens are weakly acidic because the conjugate base, called an enolate, is stabilized though conjugation with the π orbitals of the carbonyl.
What is the difference between ester and ketone?
A ketone has a molecular structure that includes a carbonyl bonded to carbons while an ester has a molecular structure in which a carbonyl is bonded to an alkoxy group.
Why do ketones have a high reactivity?
This is due to the very polar nature of the carbonyl group which in turn gives the molecule a partial positive charge. This means that other substances are often attracted to the molecule with which they then readily react. In fact, nucleophilic addition and oxidation and reduction reactions are common.
What is an ester?
Definition: An ester is a molecule which has a carbonyl group and is a molecule in which the hydroxyl has been replaced by a group that is known as an alkoxy (an oxygen atom and an alkyl group).
What are some examples of ketones?
There are many examples of ketones including acetone, phenylethanone, and propanone. Ketones form the basis for the production of many useful products in industry. For example, they are often used to make various solvents such as acetone that is widely used as a nail polish remover due to its properties as an excellent solvent. Ketones can also be used to strip paints and lacquers and even to make explosives and are often used in the tanning industry.
What is the process of forming a ketone?
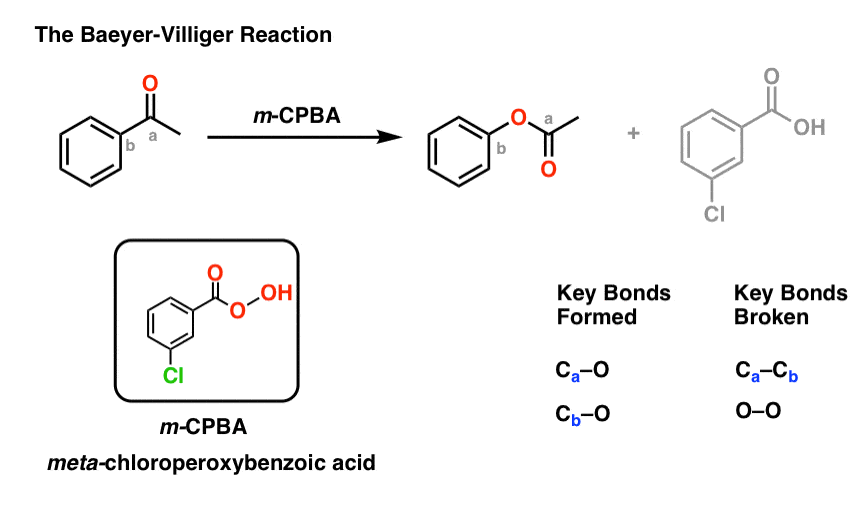
Formation. The oxidation of alcohols and hydrocarbons can produce a ketone as can the hydration of alkynes. The esterification reaction in which carboxylic acid and water are reacted can produce an ester.
How are esters formed?
Formation: Esters can be formed from alcohols, carboxylic acids and certain of the inorganic acids such as nitric acid or phosphoric acid. The process in which carboxylic acid reacts with water in the presence of some type of acid in the presence of heat to form an ester is known as esterification.
What is esters used for?
Some esters are used to make PVC wire insulation since it makes the insulation flexible. Many tricarboxylic acid esters can be found in wine and help to gives wine a specific smell. Esters are used in the manufacture of many substances including Plexiglas and Mylar film.
What is the difference between ester and ketone?
The key difference between ketone and ester is that ketone has a carbonyl functional group whereas ester has a carboxylic acid functional group. Ketones and esters are organic compounds which differ from each other according to the functional group they contain. Also, a notable difference between ketone and ester is their smell.
How to name a ketone?
Thus, the ketone is named by changing the suffix of the parent alkane from –ane to –anone. For example, a ketone having three carbon atoms and a carbonyl group at ...
How does an ester form?
An ester forms when the hydrogen atom of a carboxylic acid is replaced with an alkyl or aryl group. We can obtain esters from either carboxylic acids or alcohols. In the nomenclature of an ester, a compound gets its name according to the name of the parent compound (alcohol or carboxylic acid).
What is the formula for a ketone?
Furthermore, the general formula of a ketone is RC (=O)R’ and for ester it is RCO2R′. When considering the polarity, esters are more polar than ketones, and they are more volatile as well. So, we can consider this too as a difference between ketone and ester. Moreover, their specific smell is an easily distinguishable difference between ketone ...
Which is more volatile, carboxylic or esters?
They are more volatile than carboxylic acids of the same weight. Esters are the components in fruits that are responsible for the fruity aroma. Fruits that have esters include apple, durian, pineapple, pears, strawberry, etc. Moreover, the fats in our body are triesters derived from glycerol and fatty acid.
What is the suffix for ester?
In the name of ester, we use the suffix –oate. It has two words in its name, which gives the name of alkyl (or aryl) group attached to the oxygen atom of the carboxylic acid functional group followed by the name of alkyl group attached to the carbon atom of the functional group (with the –oate suffix).
Can carboxylic acid be treated with alcohol?
Here, we need to treat carboxylic acid with an alcohol in the presence of a dehydrating agent. Moreover, we can produce this compound via esterification of carboxylic acids with epoxides, alkylation of carboxylate salts, carbonylation, etc.
Which resonance structure removes the positive charge from the carbonyl carbon?
Both the ketone and ester have a resonance structure that places positive charge on the carbonyl carbon, however you can also draw a third resonance structure for the ester that removes some of the positive charge from the carbonyl carbon and places it on the ester oxygen.
Is LUMO more accessible than ester?
So the LUMO of the ketone will be more accessible compared to the ester (smaller energy difference). Another important fact is, that the ester is much more rigid than the ketone as it has one low lying MO that spans the O = C − O moiety (MO20), which is obviously not present (as it cannot be) in the ketone (MO18).
Is ester carbonyl positive or negative?
This makes the ester carbonyl carbon less positive (more negative, more nucleophilic, less prone to attack by a nucleophile) than the ketone carbonyl carbon (more positive, less nucleophilic, more prone to attack by a nucleophile).
Is carbonyl a nucleophile?
The este r carbonyl carbon is a stronger nucleophile and less prone to nucleophilic attack than the carbonyl carbon in a keto ne. I think you are trying to understand why the carbonyl in a ketone typically reacts faster with a nucleophile than the carbonyl in an ester. Look at the resonance structures drawn below.
Is ester a nucleophile?
I guess the ester is a weaker nucleophile because it does have an additional oxygen atom, unlike the ketone, that is pulling electrons from the C-O double bond towards the carbon atom (this happens vice versa too of course with the ester oxygen). This lowers the ionic character of the C-O double bond ...
Is LUMO lower than ester?
The LUMO of the ketone has a slightly lower ( − 1.57 e V) energy than that of the ester ( − 0.87 e V ). More important, the coefficient in this orbital is larger at the carbonyl carbon in the ketone compared to the ester. And this orbital will be attacked by the nucleophile, which usually has a high lying HOMO.