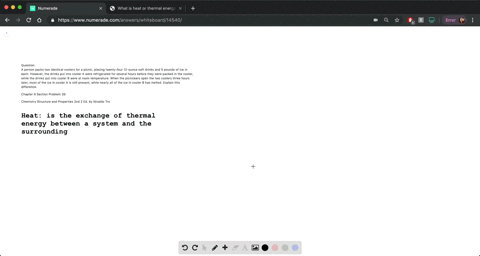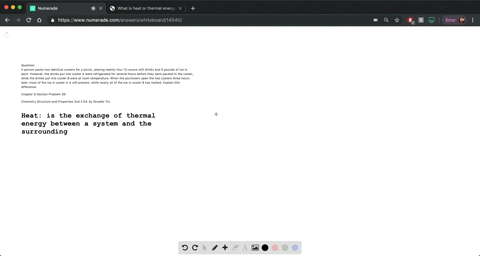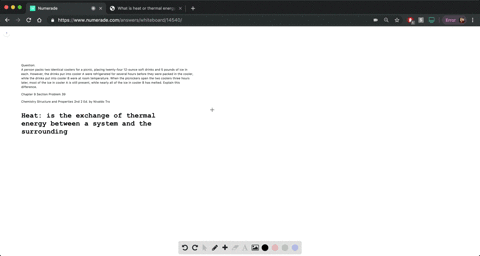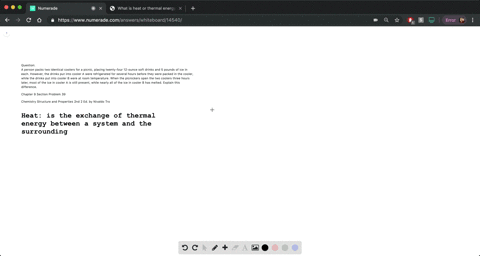
Why does a balloon shrink when it is inflated?
This makes the molecules move more slowly and have less frequent and weaker collisions with the inside wall of the balloon, which causes the balloon to shrink a little. Click to see full answer.
What happens to helium balloons in cold weather?
Boyle's Law states that if the temperature remains the same and the pressure changes, the volume of the gas in the balloons will decrease as pressure is increased and will increase as pressure is decreased. Do helium balloons last longer in heat or cold? Helium is sensitive to temperature changes.
Why do balloons sink to the ground at room temperature?
This decreases the volume inside the balloon and makes the shell of the balloon shrink and sink to the ground. Beside above, why do balloons return to normal at room temperature? When it is warmed up, the balloon regains its original volume and shape.
What happens to a balloon when it is frozen?
The frozen balloon shrank because the average kinetic energy of the gas molecules in a balloon decreases when the temperature decreases. This makes the molecules move more slowly and have less frequent and weaker collisions with the inside wall of the balloon, which causes the balloon to shrink a little. Click to see full answer.
Does cold air make balloons shrink?
“The reason that the balloons deflate in the cold weather is because the helium is denser than the air,” Coulter said. “So when it gets cold the molecules will gather together and make the balloon have less volume. When the temperature is colder, the pressure will also decrease, but the density will increase.
What happens to air filled balloons in cold weather?
This happens because the cold air causes the helium or air molecules to become more compressed and therefore the balloon begins to deflate.
Why does the balloon shrink when placed in cold water?
(Gases always expand when they're warm - the heat gives the gas energy to spread out more). The expanding gas blows up the balloon. When you put the bottle into cold water, the air cools down again. Cool air hasn't got as much energy, so it shrinks - and the balloon shrinks with it.
Do balloons last longer in cold?
The longer they are in the cold the more molecules that can escape. If a balloon is left out in the cold for too long before being brought back into the warm the number of molecules in the balloon will have reduced.
At what temperature do balloons deflate?
WHY DO FOIL BALLOONS LOOK LIKE THEY ARE DEFLATING IN THE COLD? – The helium gas starts to contract around the temperature of 50-45 degrees and will decrease in volume. Once the balloon is in a warmer place the balloon will coming back to its normal size.
Why do balloons shrink?
The lighter helium gas molecules leave the balloon faster than the heavier nitrogen and oxygen molecules can enter the balloon. Over a period of time, the balloon shrinks!
What caused the balloons to expand and shrink?
In a balloon, cooling the gas inside would cause the circumference to decrease. Answer 3: If a balloon is heated up, the gas inside will expand, causing the circumference of the balloon to increase. Similarly, if the balloon is cooled down, the gas inside contracts, and causes the balloon to shrink.
Do balloons shrink in the heat?
Helium is sensitive to temperature changes. Cold air causes the helium to shrink, which makes the balloon appear to deflate, although it still floats. Heat can cause the helium to expand and the balloon to burst. Latex balloons are also sensitive to light, and balloons of any kind are weakened by dirt and dust.
Why does a frozen balloon shrink?
The frozen balloon shrank because the average kinetic energy of the gas molecules in a balloon decreases when the temperature decreases. This makes the molecules move more slowly and have less frequent and weaker collisions with the inside wall of the balloon, which causes the balloon to shrink a little. Click to see full answer.
What happens to a balloon when it is warmed up?
When it is warmed up, the balloon regains its original volume and shape. Explanation 1: The volume of the balloon decreases by the low temperature, because the gas inside is cooled down. At room-temperature the helium balloon is lighter than air with the same volume of the air, which makes it go up. In this way, what happens to a balloon ...
What happens to the volume of helium balloons as the temperature drops?
As the temperature drops, the volume decreases. As the temperature drops, the volume will lessen. Boyle's Law states that if the temperature remains the same and the pressure changes, the volume of the gas in the balloons will decrease as pressure is increased and will increase as pressure is decreased. Do helium balloons last longer in heat ...
Why do balloons deflate?
Helium is sensitive to temperature changes. Cold air causes the helium to shrink, which makes the balloon appear to deflate, although it still floats. Heat can cause the helium to expand and the balloon to burst. Latex balloons are also sensitive to light, and balloons of any kind are weakened by dirt and dust.
Does cold air shrink balloons?
Click to see full answer. Accordingly, does cold air make balloons shrink? Cold air doesn't cause latex helium-filled balloons to deflate, but it does make helium molecules lose energy and move closer together. This decreases the volume inside the balloon and makes the shell of the balloon shrink and sink to the ground.