Does aspirin dissolve faster in water or vinegar?
The ingredients of Disprin are as follows:
- Aspirin (API or Active Pharmaceutical Ingredient)
- Calcium carbonate (Bulking agent)
- Maize starch (Bulking agent/Lubricant/Glidant)
- Citric acid (Organoleptic excipient to improve taste for increased patient compliance)
- Talc (Again a bulking agent/glidant)
- Sodium lauryl sulphate (Surfactant)
- Saccharin (Sweetener or organoleptic excipient)
Does aspirin dissolve faster in water?
Think of it simply and basically: hot temperatures melts objects. The aspirin didn’t melt, but the hot water made it less solid, therefore it expanded. That expansion allowed more water to be exposed simultaneously to the same amount of aspirin, dissolving it faster.
Why is aspirin soluble in alcohols but not in water?
Aspirin
- 1 Structures
- 2 Names and Identifiers. Computed by LexiChem 2.6.6 (PubChem release 2019.06.18) Computed by InChI 1.0.5 (PubChem release 2019.06.18)
- 3 Chemical and Physical Properties. ...
- 4 Spectral Information. ...
- 6 Chemical Vendors
- 7 Drug and Medication Information
- 8 Pharmacology and Biochemistry. ...
- 9 Use and Manufacturing. ...
- 10 Identification. ...
- 11 Safety and Hazards. ...
Does aspirin dissolve faster in hot or cold water?
Yes | No Aspirin dissolves faster in hot water because the molecules begin to move faster and starts to break down faster. source: Does aspirin dissolve faster in hot or cold water?
Is aspirin soluble or insoluble in water?
Aspirin is a non-polar molecule which is insoluble in water in its molecular form. The molecular form of aspirin reacts as a carboxylic acid, and will form a water-soluble salt upon its reaction with sodium carbonate.
Is aspirin soluble in water Yes or No Why?
Aspirin is slightly soluble in water: the solubility of aspirin in water is 0.33 grams per 100 mL water at room temperature.
Why is aspirin soluble in organic solvents?
Mechanism responsible for solubility enhancement through cosolvency is by reducing the interfacial tension (polarity differences) between the aqueous solution and hydrophobic solute. The aspirin molecule is made up of a benzene ring, a carboxyl group, and an ester. It has both polar and non-polar components.
Is aspirin soluble in cold water?
Because aspirin is less soluble in cold water. In fact, aspirin is not very soluble in water at all, which is why you are supposed to take it with lots of water. You are not "chilling the aspirin", you are preventing it from dissolving too much so that you have a better yield of your product.
How is aspirin soluble?
Aspirin contains polar functional groups which can form hydrogen bonds with polar water molecules. Aspirin is more soluble in basic (alkaline) solutions, so it readily dissolves in the duodenum which is the first part of the intestine.
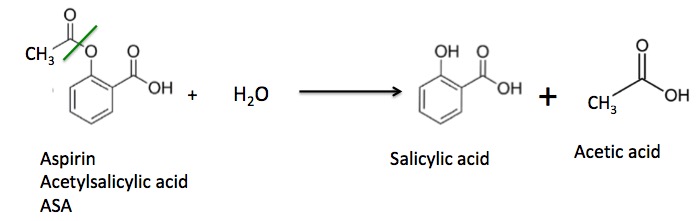
Does aspirin react with water?
Aspirin (acetylsalicylic acid) reacts with water (such as water in body fluids) to give salicylic acid and acetic acid, as shown in Figure 5.2.
Is aspirin hydrophobic or hydrophilic?
hydrophobicAspirin and salicylic acid are hydrophobic compounds. When aspirin is exposed to water or moisture it will begin to hydrolyze, resulting in salicylic acid and acetic acid.
Is aspirin polar or nonpolar?
polarAspirin is a polar molecule with dipole-dipole attraction bonds and an -OH (hydroxyl) segment as part of a carboxylic acid group. This makes it easily dissoluble in other polar liquids, such as water (H2O) and blood plasma.
Why is aspirin insoluble in hexane?
Acetylsalicylic acid is fairly polar and not soluble in pure hexane. Ethyl acetate (CH3CO2CH2CH3) is much more polar and your compound is very soluble in pure ethyl acetate.
Is aspirin soluble in hot water?
(Aspirin, like many other substances, is more soluble in hot water than in cold water. Therefore, to maximize the amount of crystals, it is best to cool the mixture as much as possible.)
Why is aspirin soluble in hot water?
Energy from hot water molecules makes solids more soluble. In hot water, molecules are moving around more, so there are more collisions between the water molecules and a solid.
Does aspirin dissociate in water?
Aspirin is a pain reliever and fever reducer, but if it's allowed to react with water then it can undergo hydrolysis, forming salicylic acid and acetic acid, which is no longer effective. This reaction can occur under acidic or basic conditions.
Why does aspirin smell like vinegar?
This is because the process of hydrolysis produces acetic acid. In addition to treating pain and fever, aspirin is used as an anticoagulant treatment to prevent blood clots and heart attacks.
Is aspirin an anticoagulant?
In addition to treating pain and fever, aspirin is used as an anticoagulant treatment to prevent blood clots and heart attacks. It also shows promise in the prevention of certain cancers, such as those of the lower gastrointestinal tract. ADVERTISEMENT.
Is aspirin soluble in water?
Jost Hiller/StockFood Creative/Getty Images. Aspirin is slightly soluble in water. It is also mildly soluble in acidic solutions, including gastric juices. It is more soluble in basic solutions, which make it easily dissolvable in the human intestines. Aspirin is considered a weak acid, and when it is dissolved in water or when it becomes old, ...
Which group of molecules can form favourable hydrogen bonds with carboxylic acid and dipole interactions with the ester group
In ethanol, the alcohol group can form favourable hydrogen bonds with carboxylic acid and dipole interactions with the ester group. The ethyl side chain, which is a hydrocarbon side chain, can form VDW forces with hydrophobic benzene. Matt Laine.
Is aspirin soluble in hot water?
So, in conclusion. aspirin is soluble in hot water and slightly soluble in ice-cold water and ranging in between the two extremes as the temperatures range between boiling and freezing points. Related Answer. Martin J Pitt. , Former Chartered Chemist and Chartered Chemical Engineer.
Can aspirin be dissolved in ethanol?
On the other hand, when the same compound is placed into a less polar solvent like ethanol, then the non soluble part of aspirin has much less problems to associate with the solvent, then more aspirin can be dissolved in ethanol.
Is salicylic acid polar or nonpolar?
Salicylic acid is also slightly polar, due to it’s carboxy group and the pi-cloud of the benzene ring. Although, you might need to apply some heat. Here’s an article I found that might be useful to you. Solubility of Salicylic Acid in Water, Ethanol, Carbon Tetrachloride, Ethyl Acetate, and Xylene.
Is aspirin a polar or nonpolar solvent?
Ethanol contains both polar and non-polar component and this makes it a better solvent than water as both sides serve as solvent ( polar- hydrogen bond, non-polar- covalent) 3.3K views. ·.
What is the reaction of aspirin?
Reactions of Aspirin (acetylsalicylic acid) The neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in commercially available aspirin tablets using a back (indirect) titration method.
What acid is used to synthesize aspirin?
Synthesis of Aspirin (acetylsalicylic acid) Salicylic acid will rapidly react with acetic anhydride in the presence of an acid catalyst to produce aspirin (acetylsalicylic acid) and acetic acid (ethanoic acid). Sulfuric acid or phosphoric acid are often used to catalyse the reaction. salicylic acid. +.
Why does aspirin smell like vinegar?
Old aspirin tablets may have a smell like vinegar as a result of the hydrolysis reaction producing acetic acid (ethanoic acid).
What is the purpose of aspirin?
It is also used to help prevent heart attacks, strokes, and blood clot formation in people at risk of developing blood clots. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group.
Is aspirin soluble in water?
Aspirin is more soluble in basic (alkaline) solutions, so it readily dissolves in the duo denum which is the first part of the intestine. Ionic salts of aspirin, such as sodium acetylsalicylate, are more soluble in water since they form stronger ion-dipole interactions with water .
Can salicylic acid react with acetic acid?
Salicylic acid can react with acetic (ethanoic) acid in an esterification reaction, but the reaction is very slow, taking days to reach equilibrium, and the yield is low: salicylic acid. +. acetic acid. (ethanoic acid) [H 2 SO 4] →. aspirin. (acetylsalicylic acid)
Why does aspirin dissolve the fastest in acid? ASAP
why does aspirin dissolve the fastest in comparison to other pain relievers????
Re: Why does aspirin dissolve the fastest in acid? ASAP
I'm hoping you have had some chemistry, because the molecular interaction of the aspirin with water/acid is the best way to explain this. Solutes, such as aspirin, dissolve in acid due to the chemical composition of the binder used in the tablet and the chemical nature of the solute.