What solutions would have the lowest freezing point?
Mar 31, 2020 · 0.1mCaI2 will have the lowest freezing point, followed by 0.1mNaCl, and the highest of the three solutions will be 0.1mC6H12O6, but all three of them will have a lower freezing point than pure water.
What substance has the lowest freezing point?
Remember, the greater the concentration of particles, the lower the freezing point will be. 0.1mCaI2 will have the lowest freezing point, followed by 0.1mNaCl, and the highest of the three solutions will be 0.1mC6H12O6, but all three of them will …
Which one has the highest freezing point?
Consequently, C u ( N O 3) 2 will have the maximum depression in freezing point and hence will have the lowest freezing point. So, the correct answer is “Option C”. Note: For solutes showing association, i > 1 For solutes showing dissociation, i < 1 For solutes showing no association or dissociation (non-electrolytes), i = 1. 149.7k + views
Which refrigerant having the lowest freezing point?
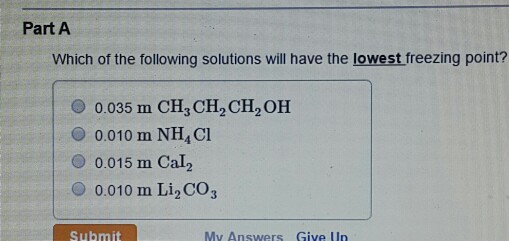
Jul 10, 2017 · In order to determine which solution has the lowest freezing point, we need to look at the molality as well as whether the solute is ionic or covalent. Since the molality is equal for all of the answer choices, we need to look at what kind of solutes are present. Choice A: NaCl is ionic since it has a metal and a nonmetal. NaCl dissociates into 2 ions: Na+ and Cl -.
Why does helium not freeze?
The reasons why are dictated by quantum mechanics: the zero point energy of a helium system is too great to allow freezing. The zero point energy is the minimum energy a particle or system always has, no matter what.
Is helium a superfluid?
Some of helium’s most unusual properties can be coaxed out at temperatures close to absolute zero. At such temperatures, helium behaves as a superfluid, meaning it flows with zero measurable viscosity. It also has a tendency to creep up the walls of a container it is held in. Michael Anissimov.
Is helium a cryogenic gas?
Liquid helium is often used as a cryogenic cooling agent when liquid nitrogen isn’t enough. It must be kept under continuous high pressure and low temperature, otherwise it quickly expands and transitions to a gas. Solid helium does not have any practical applications outside of scientific research.
Does helium have a freezing point?
Helium is the only substance that does not have a freezing point under ambient pressure, no matter the temperature. A freezing point for helium only exists under at least 25 atmospheres of pressure and a temperature of 1.15 K. These conditions have been created in a laboratory through evaporative cooling.
Is helium more dense than air?
The density of solid helium itself is only 66 times greater than air. By comparison, water is 1000 times more dense than air. Helium was first liquefied in 1908 by Dutch physicist Heike Onnes, who cooled it to 1 degree Kelvin. Much to his surprise, further cooling did not cause it to reach its freezing point.
Who is Michael Anissimov?
Michael Anissimov. Michael is a longtime InfoBloom contributor who specializes in topics relating to paleontology, physics, biology , astronomy, chemistry, and futurism. In addition to being an avid blogger, Michael is particularly passionate about stem cell research, regenerative medicine, and life extension therapies.
Which has the lowest freezing point?
Remember, the greater the concentration of particles, the lower the freezing point will be. 0.1mCaI2 will have the lowest freezing point, followed by 0.1mNaCl, and the highest of the three solutions will be 0.1mC6H12O6, but all three of them will have a lower freezing point than pure water.
What element Cannot freeze?
That’s due to the fact that Helium has the lowest boiling and freezing points of any other known substance. Helium happens to be the only element that can’t be solidified or frozen at normal atmospheric pressure. Only once you apply a pressure of 25 atmospheres at Helium’s freezing point of −458 °F can you solidify it.
Do all elements freeze?
The basic idea is at low enough temperature, most of elements will freeze in the solid state; a higher temperature will make them liquid; an even higher temperature will turn them into gas. This is true for most of complex compounds as well.
At what temp does co2 freeze?
Carbon dioxide is a gas at room temperature, and it freezes solid at a much lower point than water: -109 degrees Fahrenheit (-78 C).
Which metal has the lowest melting point?
15 lowest melting point metals: Mercury, Francium, Cesium, Gallium, Rubidium, Potassium, Sodium, Indium, Lithium, Tin, Polonium, Bismuth, Thallium, Cadmium, and Lead. We also created a list of metals with the highest melting point.15 Metals With The Lowest Melting Point.
Can you eat gallium?
Although it is not harmful in small amounts, gallium should not be purposefully consumed in large doses. For example, acute exposure to gallium (III) chloride can cause throat irritation, difficulty breathing, chest pain, and its fumes can cause even very serious conditions such as pulmonary edema and partial paralysis.
What liquids dont freeze?
All that said, the only liquid that does not even freeze at the lowest possible temperature (“absolute zero”) is liquid helium. To turn that into a solid you additionally need to put it under pressure.
What is the freezing point of a pure solvent?
Answer and Explanation: Freezing point of a pure solvent depends on the amount of solute that gets dissolved in it. In order to determine which solution has the lowest freezing point, we need to look at the molality as well as whether the solute is ionic or covalent.
Is NaCl ionic or ionic?
Since the molality is equal for all of the answer choices, we need to look at what kind of solutes are present. Choice A: NaCl is ionic since it has a metal and a nonmetal.
What is depression of freezing point?
Explanation: According to colligative properties of solutes, depression of freezing point follows the number of particles produced upon solubilization.
Is LiF soluble in water?
On the other hand LiF is actually sparingly soluble in water (about 0.071 M), and cannot be dissolved at 0.65 molal. So the answer depends on whether we want to represent physical reality or answer a theoretical question. [Sucrose and glucose give only one particle per molecule.]