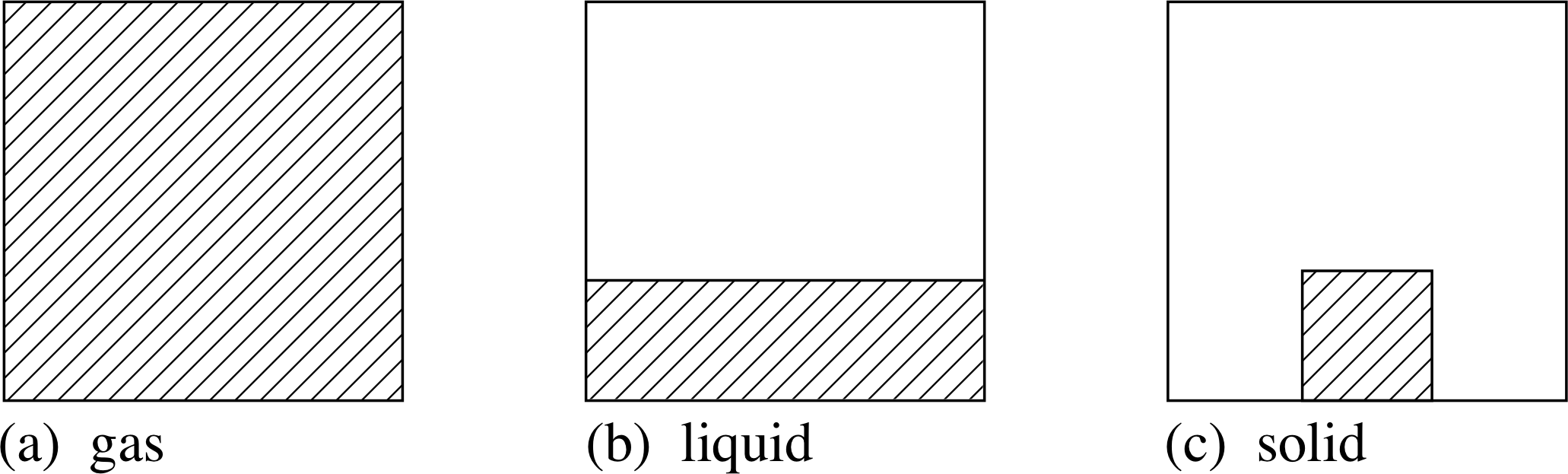
Which state of matter is the least dense?
The states of matter found on Earth include solids, liquids, and gas. Gas is the least dense state of matter as the particles are far apart. After gas, liquid is the least dense.
What state of matter has a low density and high compressibility?
The gas state, for example is a state in which the matter has indefinite shape and volume, it also has a very low density and a high compressibility. A state of matter with low density and high compressibility? Gas. What state of matter has a low density and an indefinite volume? Gaseous. Why is density an important physical property of matter?
Does high or low density have more matter?
high density have more matter per unit of volume (a box with a lot of circles) low has fewer matter per unit volume (a box with less circles) Does sodium have a high density?
What is the density of dark matter in the Milky Way?
It is about the equivalent of 2.5 protons per cubic meter, on average throughout the universe. But we do not know what the mass of individual dark matter particles is and thus the numeric density is unknown. In the plane of the Milky Way galaxy it is though to be significantly higher, perhaps 1/3 of a proton equivalent per cc.
Which solid has lowest density?
graphene aerogelThe world's least dense solid is a graphene aerogel with a density of just 0.16 mg/cm³; produced by a research team from the Department of Polymer Science and Engineering lab at Zhejiang University, China, headed up by Professor Gao Chao (China).
Which of these has lowest density?
HydrogenHydrogen is the element with the lowest density in the periodic table and exists as a gas and osmium is the element with the highest density.
Which has low density solid liquid or gas?
Gases have lower density compared to that of solids or liquids.
What state of matter has high density?
Solids also have a high density, meaning that the particles are tightly packed together. In a liquid, the particles are more loosely packed than in a solid and are able to flow around each other, giving the liquid an indefinite shape.
What is a low density?
Definition: Density depends on how atoms or particles are arranged in matter in a specific volume. If the particles are loosely packed together with plenty of space between them, it will have a low density and will be able to float.
What is less dense?
Students should realize that if an object weighs more than an equal volume of water, it is more dense and will sink, and if it weighs less than an equal volume of water, it is less dense and will float.
Is gas low density?
Gases have a lower density than other states of matter, such as solids and liquids. There is a great deal of empty space between particles, which have a lot of kinetic energy and aren't particularly attracted to one another.
Does liquid have low density?
Liquids generally have lower density as compared to solids but ice floats on water.
Which gas has the lowest density Why?
Therefore CH4 has lowest density.
Which object has the highest density lowest density Why?
Iron is more dense This is so because if we divide each object's mass by its volume, and since all three objects have the same volume, the object with the larger mass will have the greatest density. And the object with the smaller mass, will have the lowest density.
Which has more density gas or solid?
In general, solids are denser than liquids, which are denser than gases. . The particles in the solid are touching with very little space between them.
Which one has more density?
∙Iron has the highest density as the particles are closely packed and volume occupied is less which makes the density very high.
What are the states of matter?
The various kinds of substances that made up matter can be divided mainly into four states like solid, liquid, gases, and plasma. We observed these states by daily human experience. Two types of opposing molecular forces derive the states of matter. One is molecular forces of attraction which tend to hold the molecules together. Another is disruptive forces due to the thermal energy of the molecules.
What are the characteristics of gaseous states?
In gaseous states, the matter has the property to filling any available space to a uniform density. Low density and high compressibility also the characteristics of gases in learning chemistry or physics. If the thermal energy much greater than the forces of attraction, then we find the gaseous state of matter. Molecules in a gaseous state move at very high speeds.