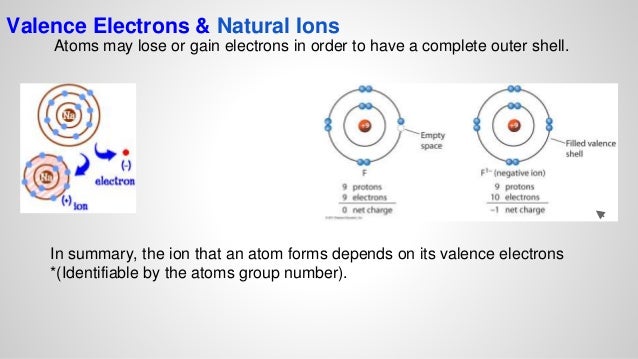
Which element would fluorine form an ionic bond with?
Sodium has 11 electrons in it, which can be written as 2,8,1. Here Sodium transfers its one electron from itself to Fluorine and thus an ionic bond is formed between them. An ionic bond is also known as electrovalent bond. Thus NaF is an ionic compound or an electrovalent compound. Click to read in-depth answer.
Does fluorine usually form ionic or covalent bonds?
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. How is an ionic bond formed between magnesium and fluorine?
Which will form an ionic bond?
which element when combined with fluorine would most likely form an ionic compound
- Determine whether the following pairs of elements can form ionic compounds MasteringChemistry HW012
- Writing Ionic Formulas: Introduction
- Ionic Compounds
Is an ionic bond stronger than a covalent bond?
Why is covalent bond stronger than ionic? Ionic bond is much stronger than covalent bond because it involves complete transfer of electrons because of which there is formation of cation and anion and there exist huge electrostatic forces of attraction. … Covalent bond is not as strong as ionic bond.
See more
Which element forms an ionic bond with fluorine quizlet?
To form ionic bond with fluorine which has the highest electronegativity, element needs to have low electronegativity. Because bond is more ionic the higher the electronegativity difference. Metals have lower electronegativities than nonmetals, and the only possible answer that is metal is potassium.
Does fluorine make ionic bonds?
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds.
What is the most ionic form of fluorine?
sodium fluorideAs fluorine is highly electronegative than other atoms and smallest in size, sodium fluoride is more ionic than other molecules.
Which element will form an ionic bond with a halogen?
For example, alkali metals in group 1 form ionic bonds with halogen nonmetals in group 17.
Are potassium and fluorine an ionic bond?
Now, after accepting one electron from potassium fluorine has eight electrons in its outermost shell to get stable. This will produce potassium cation and fluorine anion. This oppositely charged ions will result in electrostatic attraction which we call it as ionic bond.
Is lithium and fluorine ionic?
By accepting one electron from lithium, fluorine is able to achieve the electron configuration of the noble gas neon. So, the ionic bond formed between lithium and fluorine increases the chemical stability of the atoms.
How many bonds can fluorine form?
one bondIt has 9 electrons, 2 core and 7 valence. Rather than forming 7 bonds, fluorine only forms a single bond for basically the same reasons that oxygen only forms two bonds. Hydrogen fluoride, HF, has one bond, but four centers of electron density around the fluorine.
What type of bond is fluorine?
covalent bondFluorine and the other halogens in group 7A (17) have seven valence electrons and can obtain an octet by forming one covalent bond....How Many Covalent Bonds Are Formed?Atom (Group number)Number of BondsNumber of Lone PairsOxygen (Group 16 or 6A)22Fluorine (Group 17 or 7A)132 more rows•Jul 1, 2019
What are the compounds formed by fluorine?
Sodium fluoride (NaF), stannous(II) fluoride (SnF2) and sodium monofluorophosphate (Na2PO3F) are all fluorine compounds added to toothpaste, also to help prevent tooth decay. Hydrofluoric acid (HF) is used to etch glass, including most of the glass used in light bulbs.
Does F and Al form an ionic compound?
As Al is a metal so it will form ionic compound AlF3 with fluorine while P forms covalent compound with F.
What elements can form ionic bond?
Ionic compounds generally form between elements that are metals and elements that are nonmetals. For example, the metal calcium (Ca) and the nonmetal chlorine (Cl) form the ionic compound calcium chloride (CaCl2).
Which elements are more likely to form an ionic bond?
An ionic bond is most likely to form between metal and nonmetal elements. An element can be classified as a metal if it's found on the left side of the periodic table, while nonmetals are located on the right side of the periodic table.
What is the central idea of an ionic bond?
In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings (shells) of adjacent atoms. 1) For example, consider Na and Cl. Sodium would lose one electron and become positively charged and the chlorine would gain one electron becoming negatively charged.
What would hold two ions together?
The positive/negative charge attraction would hold the two ions together. 2) Another example, magnesium and oxygen. The magnesium would lose two electrons, becoming +2 charged and the oxygen would gain the two electrons becoming -2 charged in the process.
Is hydrogen an ionic bond?
Hydrogen can be involved in ionic bonding. It will act as a nonmetal with anegative one charge. It is named hydride. There are more complex ionic bonding situations which will remain for later. For example, the bond between NH4+and Cl¯ in ammonium chloride is an ionic bond. Return to Bonding menu.
What is the ionic charge of an atom?
Ionic Charges of All Elements (List) Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is known as Ionic charge. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, ...
What is the charge generated by an ion?
This electric charge generated on the ion is known as Ionic charge. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed.
How many valence electrons does fluorine have?
Jul 12, 2018. A potassium atom has one valence electron in its outermost (fourth) energy level, and fluorine has seven valence electrons in its outermost (second) energy level.
Which ionic bond is formed when the oppositely charged ions are opposite?
The oppositely charged ions form an electrostatic attraction, which is the ionic bond. The compound potassium fluoride (KF) results, and since the potassium and fluoride ions have equal but opposite charges, the compound is neutral (but not the individual ions in the compound).