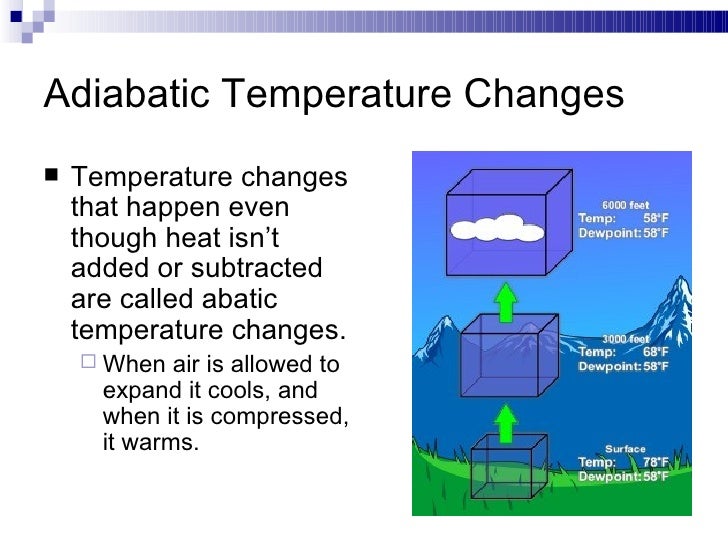
The increase in temperature happens because the parcel is being compressed by increasing air pressure as it moves downward. If there is liquid water in the parcel (cloud droplets or raindrops), the liquid will evaporate because as the parcel warms its capacity for water vapor increases.
When air is compressed rapidly the temperature increases?
When air is compressed rapidly, the temperature increases according to the laws of thermodynamics. Compression of air is caused under increased pressure, which leads to rise a proportional increase in heat.
What happens to the temperature as the air parcel rises?
The air parcel expands as it rises and this expansion, or work, causes the temperature of the air parcel to decrease. As the parcel rises, its humidity increases until it reaches 100%.
What is compression of air under increased pressure?
Compression of air is caused under increased pressure, which leads to rise a proportional increase in heat. The increase in heat leads to a rise in temperature of the system. Gay-Lussac's law states that the pressure of the gas is directly proportional to temperature at constant temperature.
What is the relationship between temperature and pressure in a gas?
The increase in heat leads to a rise in temperature of the system. The pressure of the gas is doubled during compression so that the volume of air gets halved as per Boyle's law: The volume of the gas is inversely proportional to pressure at constant temperature.
What happens to temperature when air is compressed?
Pressure multiplied by volume divided by temperature equals a constant. The combination law explains what happens to air when it's compressed into a smaller volume. It tells us that when air is compressed, the pressure and temperature of the air increases, as the volume of the space containing air decreases.
What happens to temperature when an air parcel rises?
A parcel of air expands and becomes less dense as it rises. This occurs because the air pressure lowers around the parcel as it increases in altitude. The volume of the parcel increases since it is expanding. The temperature of a rising parcel always cools even though it is becoming less dense.
How does compression increase temperature?
Solution : Sudden compression of a gas is an adiabatic process. The work done in compressing the gas increases the internal energy of the gas. Hence the temperature of the gas rises.
How does the temperature of a gas change when it is compressed when it expands?
As a gas (like air) expands, the value of V increases and this has the effect of increasing T (The temperature). As the energy needed to increase it's temperature must be supplied from somewhere, the gas takes the energy from the surrounding system giving the effect of cooling.
What happens when an air parcel rises?
The air parcel expands as it rises and this expansion, or work, causes the temperature of the air parcel to decrease. As the parcel rises, its humidity increases until it reaches 100%. When this occurs, cloud droplets begin forming as the excess water vapor condenses on the largest aerosol particles.
Why do air parcels cool as they rise?
A rising parcel of air expands because the air pressure falls with elevation. This expansion causes the air to cool.
Does temperature increase with pressure?
In a closed system where volume is held constant, there is a direct relationship between Pressure and Temperature. In a direct relationship, one variable follows the same change when it comes to increasing and decreasing. For example, when the pressure increases then the temperature also increases.
What happens when you compress gas?
During compression, the volume (V) of a gas decreases. When this happens, the pressure (P) of the gas increases if the number of moles (n) of gas remains constant. If you keep the pressure constant, reducing the temperature (T) also causes the gas to compress.
Does compressed gas heat up?
The reason why a gas heats up when it is compressed into a smaller space, is because the ambient heat that the gas possessed in its original volume, has now been confined to a smaller volume—same amount of heat but now more concentrated—the temperature goes up.
When air is quickly compressed why does its temperature increase?
The air's temperature increased when quickly compressed because work was done on the air so its internal energy increased causing an increase in temperature.
What happens to the temperature of a gas when it is compressed quizlet?
What happens to the temperature of a gas when it is compressed? The temperature increases.
Why does temperature increase when compressed?
When air is compressed rapidly, temperature increases because temperature and volume of gas are inversely proportional to each other according to gas laws. When air is compressed rapidly, its volume decreases leading to increase in temperature.
What happens to the temperature of a gas when it increases?
The increase in heat leads to a rise in temperature of the system. The pressure of the gas is doubled during compression so that the volume of air gets halved as per Boyle's law: The volume of the gas is inversely proportional to pressure at constant temperature.
Which law states that the volume of a gas is directly proportional to the temperature of the gas?
Gay-Lussac's law states that the pressure of the gas is directly proportional to temperature at constant temperature. According to Charles law, the volume of the gas is directly proportional to temperature at constant pressure.
What is the equation for the gas law?
The combined gas law gives the relation between pressure, temperature, and volume of gas as follows: PV/T = constant. Therefore, the combined gas law equation indicates that the pressure of gas is directly proportional to temperature. As the pressure exerted on the air increases during compression, the temperature of air also increases.