Discovery and Features of Subatomic Particles
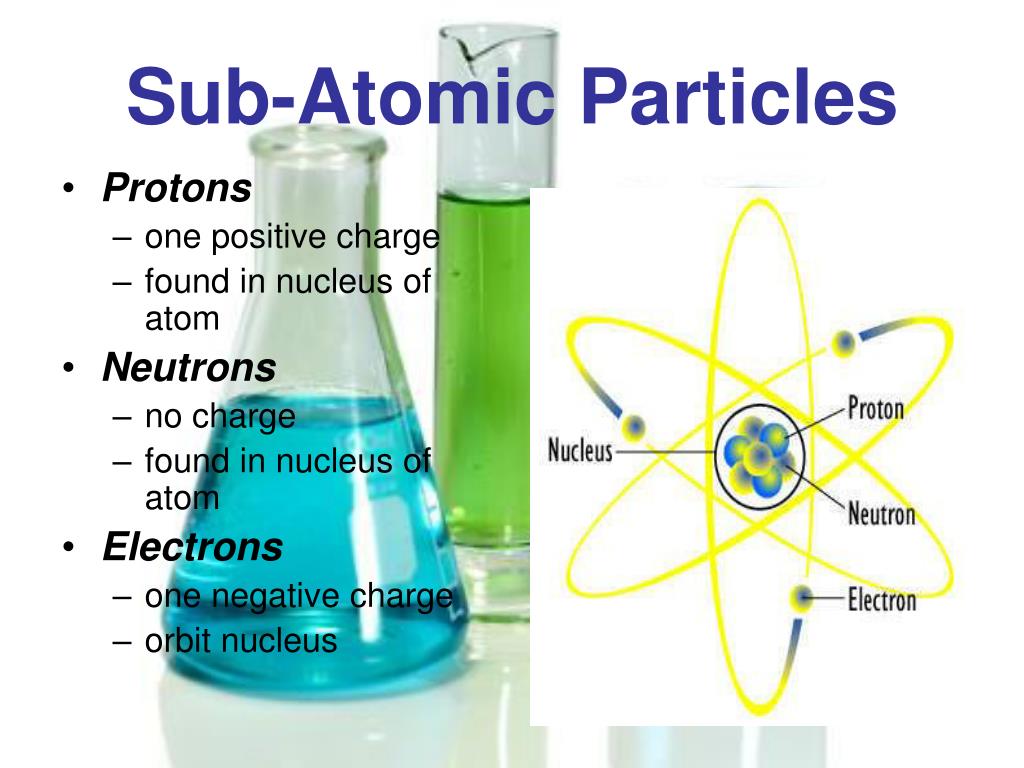
- Protons. Protons and Neutrons together make up the nucleus of an atom and are hence called nucleons. Some important points regarding the discovery and properties of protons are listed below.
- Electrons. Electrons are the subatomic particles that revolve around the nucleus of an atom. ...
- Neutrons. Neutrons, along with protons, make up the nucleons. ...
What is the subatomic particle of an atom?
The subatomic particle, of course, is the electron, which orbit the nucleus of the atom by traveling in energy levels, two in the first, 6 in the second, and 10 in the third. It is the number of electrons available in the outer energy level that determine the atoms chemical properties.
What particle is responsible for the chemical properties of an atom?
Originally Answered: What particle is responsible for the chemical properties of an atom? None really. The chemical properties of an element determine the way that element interacts with other elements and conditions in the environment on a macro level of scale.
What is the discovery and features of subatomic particles?
Discovery and Features of Subatomic Particles. The discovery of the three basic subatomic particles and some of their important features are discussed in this subsection. Protons and Neutrons together make up the nucleus of an atom and are hence called nucleons.
What determines the chemical properties of an atom?
The chemical properties of elements depend on an element's electron configuration. When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react. The chemical properties of an element depend on the number of valence electrons. What determines the chemical properties of an atom quizlet?
Which subatomic particle is responsible for chemical properties of atoms?
Answer and Explanation: The chemical properties of an element are determined by the subatomic particle known as a proton.
What determines the chemical property of an atom?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons.
Which of the following subatomic particles determines the chemical element?
The identity of the element is determined by the number of protons, which are positively charged, massive nuclear particles. The number of protons in a nucleus is given by Z , the atomic number.Sep 14, 2016
Which subatomic particles are involved in chemical bonding?
Of the three subatomic particles,electrons are most involved in forming chemical bonds.
What subatomic particle makes the element unique?
Every element has its own unique number of protons, and therefore, atomic number.Jul 27, 2017
What are the subatomic particles?
The three subatomic particles are protons, neutrons and electrons. Protons and neutrons are found in the nucleus and electrons exist in a “cloud” outside of the nucleus and consist of the rest of the atom, as nebulous as that may be. An atom is mostly empty space.
Why is chemistry defined by protons?
It might feel more fundamental to say chemistry is defined by the protons, because which element depends on the number of protons in the nucleus. The nuclear charge sets up the potential that the electrons are the bound in. However, this would suggest that every element should be different.
What determines the number of electrons in a neutral atom?
In a chemical reaction, it is the electrons that are lost, gained, or shared. The number of protons in the nucleus determines the number of electrons in the neutral atom. The electrons orbit the nucleus in specific patterns.
How many subatomic particles can be removed?
There are only three subatomic particles that stably exist in atoms that can be removed. In taking away an electron, a positive ion is produced, and the result is that the atom becomes more chemically active, i.e., it is more likely to undergo chemical reactions when near other atoms and molecules.
Why do electrons have a charge of -1?
An electron has all its energy planes share their center point as the center point of the particle so the planar energy in an electron exist as conically shaped planes and therefore the energy of the electron exists in all three directions of spacetime. This is why an electron has a charge of -1.
What is the purpose of an atom's electrons?
An atom is mostly empty space. Sometimes it is said that electrons are responsible for bonding (although that’s not completely true) and bonds store energy (not completely true, either). Chemical bonds result from the attraction of the nucleus of each atom with all of the neighboring electrons.
What happens if you take away a neutron?
If you take away a neutron, the atomic number stays the same, meaning that the chemical nature doesn't change much, but you have a different isotope of the original element. This new atom might be radioactive. Take away a proton, and you have an isotope of a different element with an atomic number t. Continue Reading.
What are the subatomic particles?
Typically, an atom can be broken down into three subatomic particles, namely: protons, electrons, and neutrons.
What are the components of an atom called?
Further investigations revealed the existence of neutrons. The components of atoms are called subatomic particles and generally include the proton, electron, and the neutron.
What are protons and neutrons?
Protons and Neutrons together make up the nucleus of an atom and are hence called nucleons. Some important points regarding the discovery and properties of protons are listed below. Protons are positively charged subatomic particles. The number of protons in an atom is equal to the number of electrons in it. The discovery of protons is credited ...
How many protons are in an atom?
Protons can be produced via the removal of an electron from a hydrogen atom. The mass of a proton is 1.676 * 10 -24 grams. The charge of a proton is +1.602 * 10 -19 Coulombs.
Why do electrons come together?
These electrons may be removed from or gained by an atom to form ions. Electrons of different atoms come together to participate in chemical bonding. A few points detailing the discovery and the properties of electrons are listed below. Electrons are negatively charged subatomic particles.
Why are neutrons called neutrons?
Neutrons are named for their neutral nature – unlike protons and electrons, they do not carry any charge. The discovery and general properties of neutrons are discussed below. Neutrons are neutrally charged subatomic particles.
How much does an electron have a charge?
It is found to have a mass equal to (1/1837) times the mass of a proton. The charge of an electron is equal to -1.602 * 10 -19 Coulombs. Click here to learn more about electrons.