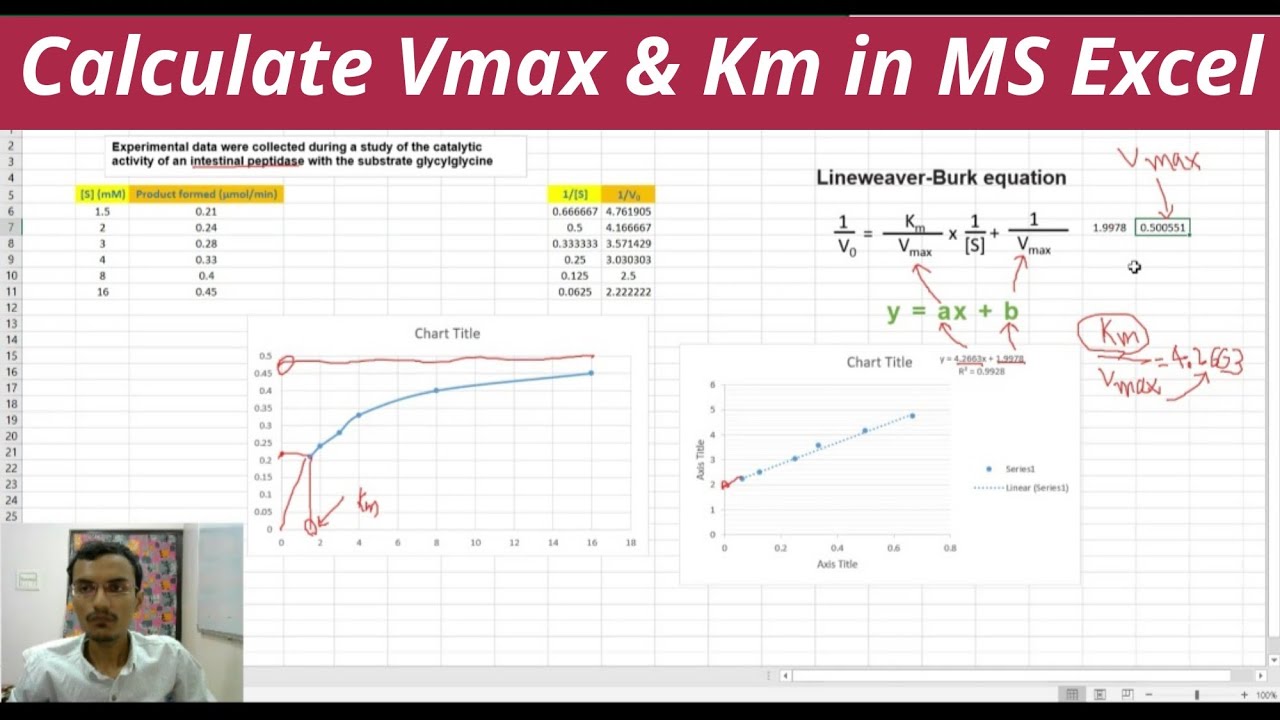
What is Vmax Km ratio? The rate of reaction when the enzyme is saturated with substrate is the maximum rate of reaction, Vmax. This is usually expressed as the Km (Michaelis constant
Michaelis–Menten kinetics
In biochemistry, Michaelis–Menten kinetics is one of the best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. This equation is called Michaelis–Menten equation. Here, represents the maximum rate achieved by t…
Is there a relationship between Vmax and km?
Vmax is the maximum rate of reaction when the substrate is saturated. Km is the Michaelis constant, which is the substrate concentration at which the reaction proceeds at half the rate Vmax. So Km determines the relationship between concentration and reaction rate, up to the point that the concentration of substrate is saturated.
How do you find kcat from Vmax and km?
How do you find kcat from Km and Vmax? TURNOVER NUMBER (kcat) – CATALYTIC CONSTANT. turnover number = kcat = Vmax/[ET] kcat/KM = catalytic efficiency. Why is lower km better? Km turns out to be the concentration of substrate required to get an enzymatic reaction to half maximum velocity.
What is the difference between kcat and Vmax?
What is the difference between Vmax and kcat? Vmax & Kcat. To determine Kcat, one must obviously know the Vmax at a particular concentration of enzyme, but the beauty of the term is that it is a measure of velocity independent of enzyme concentration, thanks to the term in the denominator. Kcat is thus a constant for an enzyme under given ...
What is km and Vmax in enzyme kinetics?
What do km and vmax values mean? Vmax is equal to the product of the catalyst rate constant (kcat) and the concentration of the enzyme. Km is the concentration of substrates when the reaction reaches half of Vmax. A small Km indicates high affinity since it means the reaction can reach half of Vmax in a small number of substrate concentration.
Is Km directly proportional to Vmax?
This is, of course not true. KM is a substrate concentration and is the amount of substrate it takes for an enzyme to reach Vmax/2. On the other hand Vmax/2 is a velocity and is nothing more than that. The value of KM is inversely related to the affinity of the enzyme for its substrate.
How do you read Vmax and Km?
5:399:54Enzyme Kinetics with Michaelis-Menten Curve - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo what km tells. You is that if you're at a lower km that means that the enzyme substrate complexMoreSo what km tells. You is that if you're at a lower km that means that the enzyme substrate complex has a very high affinity. It's easier for the substrate.
What is Km and Vmax in Michaelis-Menten equation?
The Michaelis-Menten equation for this system is: Here, Vmax represents the maximum velocity achieved by the system, at maximum (saturating) substrate concentrations. KM (the Michaelis constant; sometimes represented as KS instead) is the substrate concentration at which the reaction velocity is 50% of the Vmax.
What does higher Vmax mean?
Thus, the amount of enzyme becomes the rate-controlling parameter, and an increase in the enzymes increases the maximal velocity or Vmax. Therefore, the higher the enzyme amount, the higher the Vmax of the reaction.
What Vmax means?
The maximal velocity of the reaction (or maximal rate) Vmax is the rate attained when the enzyme sites are saturated with substrate, i.e. when the substrate concentration is much higher than the KM.
What is Vmax in enzyme kinetics?
The rate of reaction when the enzyme is saturated with substrate is the maximum rate of reaction, Vmax.
What is the significance of Michaelis-Menten constant Km?
Significance of Michaelis-Menten Constant: (i) By knowing the Km value of a particular enzyme-substrate system, one can predict whether the cell needs more enzymes or more substrate to speed up the enzymatic reaction.
Is Km half of Vmax?
By definition, the KM is the concentration in substrate that gives a rate that is EXACTLY Vmax / 2 (half the Vmax), hence the other name of Km which is half-saturation constant.
How do you read a Vmax graph?
3:385:23B7 Determine Vmax and Michaelis constant (Km) by graphical means ...YouTubeStart of suggested clipEnd of suggested clipSo let's draw this graph at again V don't forget it's velocity O which is the rate of reaction. AndMoreSo let's draw this graph at again V don't forget it's velocity O which is the rate of reaction. And here are two enzymes enzyme X and enzyme Y. Now you can read across and see that they have different
Is Km half of Vmax?
By definition, the KM is the concentration in substrate that gives a rate that is EXACTLY Vmax / 2 (half the Vmax), hence the other name of Km which is half-saturation constant.
How do you read a Michaelis Menten graph?
Explanation: In a classic Michaelis-Menten graph, the y-axis represents reaction rate and the x-axis represents substrate concentration. At low substrate concentrations, the reaction rate increases sharply.
How do you find the Km value of an enzyme?
3:136:41How to Calculate Enzyme Km using Michaelis Menten EquationYouTubeStart of suggested clipEnd of suggested clipSo just write it as km there plus substrate concentration is 2 okay now all you need to do is crossMoreSo just write it as km there plus substrate concentration is 2 okay now all you need to do is cross multiply. So 2 times km plus substrate concentration that is 2 km + 2 there.
What is the Vm/Km ratio?
Vm/Km ratio is expected to decrease when enzyme is efficiently inhibited with competitive or non competitive inhibitors and is expected to remain the same when submitted to uncompetitive inhibitor.
What is the unit of Vmax?
Vmax "represents the maximum rate achieved by the system, at maximum (saturating) substrate concentrations" (wikipedia). Unit: umol/min (or mol/s).
What is the unit of Vmax?
Vmax "represents the maximum rate achieved by the system, at maximum (saturating) substrate concentrations" (wikipedia). Unit: umol/min (or mol/s).
What is the KM of a substrate?
By definition, the KM is the concentration in substrate that gives a rate that is EXACTLY Vmax / 2 (half the Vmax), hence the other name of Km which is half-saturation constant.
Is Km a parameter?
Dominique explains this very well. It is best to regard Km and Vmax as independent parameters arising from intrinsic properties of the enzyme. Specificity is best compared from kcat/Km values. If the enzyme concentration is unknown, use Vmax/Km values for the comparison of specificities but remember that Vmax is directly proportional to the enzyme concentration so be sure that comparisons of Vmax/Km for different substrates always refer to the same amount of enzyme added to the reaction mixtures.
Is Km dependent on the Vmax?
As it is well explained to you by Dominique Liger The value of Km is absolutely dependent on the vaue of the catalytic constant k+2 which determine the Vmax when multiplied by the enzyme concentration. The Michaelis theory establishes that In the equation