What is the structural formula for ethane?
ethane, a colourless, odourless, gaseous hydrocarbon (compound of hydrogen and carbon), belonging to the paraffin series; its chemical formula is C2H6. Ethane is structurally the simplest hydrocarbon that contains a single carbon–carbon bond.
What is the correct Lewis structure for ethane?
Ethane is a gas and therefore volatilization from soil and water is expected to be the most important fate process. The Henry's Law constant for ethane is estimated as 0.5 atm-cu m/mole (SRC) derived from its vapor pressure, 3.15X10+4 mm Hg (1), and water solubility, 60.2 mg/L (2).
What are the advantages and disadvantages of ethane?
- Unlike petroleum, ethanol is a renewable resource
- Ethanol burns more cleanly in air than petroleum, producing less carbon (soot) and carbon monoxide
- The use of ethanol as opposed to petroleum could reduce carbon dioxide emissions, provided that a renewable energy resource was used to produce crops required to obtain ethanol and to ...
Does ethane have any structural isomers?
Why doesn’t ethane (C2H6) have any structural isomers? ethane has too few carbon atoms to form a branched chain. What is the maximum number of hydrogen atoms that could be bonded to an asymmetric carbon?
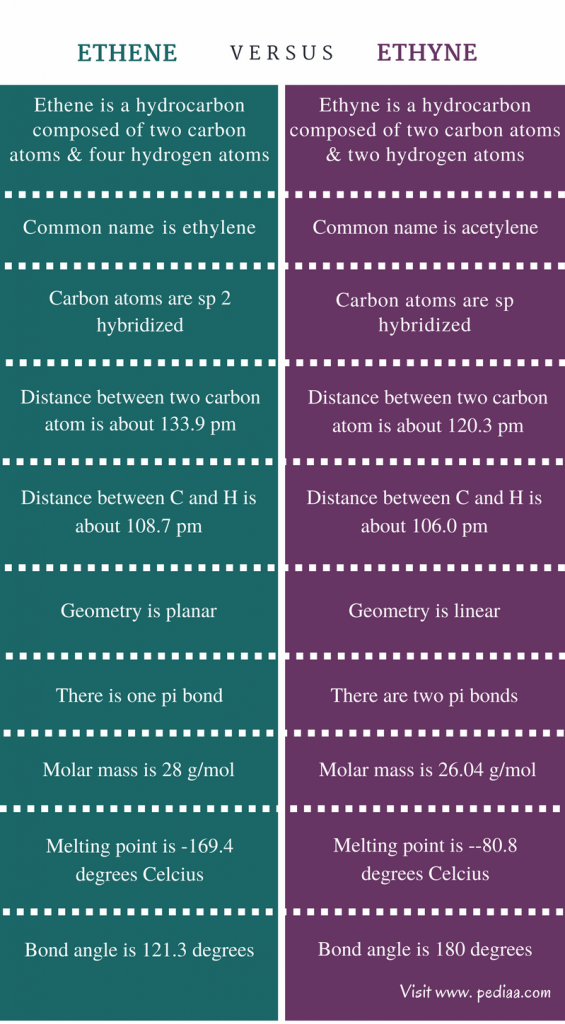
What is the structural form of ethene?
Ethylene is an unsaturated organic compound with the chemical formula C2H4. It has one double bond and is the simplest member of the alkene class of hydrocarbons. C2H4 is a simplest alkene with chemical name Ethylene. It is also called Ethene or Polyethylene or Etileno.
How do you draw the structure of ethane?
0:271:35How to Draw the Dot Structure for C2H6 - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd this carbon also has eight so that is the lewis structure for ethane c2h6. We can also draw. ItMoreAnd this carbon also has eight so that is the lewis structure for ethane c2h6. We can also draw. It as a structural formula and you'll see this quite frequently.
What is general formula of ethane?
ethane, a colourless, odourless, gaseous hydrocarbon (compound of hydrogen and carbon), belonging to the paraffin series; its chemical formula is C2H6.
What is the line bond structure of ethane?
Bonding in Ethane The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp3 orbital from each of the carbons, while the six carbon-hydrogen bonds are formed from overlaps between the remaining sp3 orbitals on the two carbons and the 1s orbitals of hydrogen atoms.
What is the structural formula of ethanol?
C2H5OHEthanol / Formula
What is the structural formula of methane?
CH₄Methane / Formula
How many atoms are in ethane?
C2H6, 2 atoms of carbon combine with 6 atoms of hydrogen to form ethane.
What is the shape of ethane?
TetrahedralWe will look at the hybridization of C2H6 (Ethane) here on this page and understand the process in detail. Students will also learn about the molecular geometry, bond formation and the bond angles between the different atoms....Hybridization of Ethane (C2H6)Name of the MoleculeEthaneGeometryTetrahedral3 more rows
What is the shape of ethene?
Hybridization of C2H4 - Ethene (Ethylene)Name of the MoleculeEthene or EthyleneMolecular FormulaC2H4Hybridization Typesp2Bond Angle120oGeometryPlanar
Q.1. What is C2H6 called?
Ans: C2H6 is called Ethane. It ranks second in the homologous alkane series.
Q.2. What is the main use of Ethane?
Ans: The main use of Ethane is that it is used in the production of Ethylene through pyrolysis or cracking.
Q.3. What does Ethane look like?
Ans: Ethane has a tetrahedron geometry with two methyl groups at each of its ends. It looks like a dimer of methyl radical.
Q.4. What is the chemical formula of methanol?
Ans: The chemical formula of methanol is CH3OH. One hydrogen atom of methane is replaced by the hydroxyl group (-OH) group.
Q.5. How can we identify Ethane from Ethene?
Ans: Ethane is an alkane and is a saturated hydrocarbon, whereas Ethene is an alkene and is unsaturated. Ethane consists of only single bonds, wher...
What is the formula for ethane?
Ethane is the second simplest alkane followed by methane. It contains 2 carbon atoms and 6 hydrogen atoms. So the formula for ethane is C 2 H 6. It is prepared by laboratory method using sodium propionate. Ethane is the most important gaseous fuel. Natural gas components of ethane and ...
How is ethane synthesized?
Ethane is synthesized by reduction of ethyl iodide using zinc + copper couple in alcohol. The chemical equation is given below. Ethane is also prepared by Wurtz reaction. When methyl bromide or methyl iodide and sodium are heated in the presence of dry ether ethane is formed.
How is ethane prepared?
It is prepared by laboratory method using sodium propionate. Ethane is the most important gaseous fuel. Natural gas components of ethane and heavier hydrocarbons are quite easily separated from the gas stream and liquefied under moderate pressure.
What is ethane used for?
It is the second most abundant natural gas component, widely used in many homes. It is also used for the production of a chemical called ethylene, which is used in the manufacture of goods such as plastic, automotive antifreeze, and detergent.
Is ethane a hydrocarbon?
Its chemical formula is C2H6. Ethane is the simplest hydrocarbon since it contains only one carbon–carbon bond in its structure.
Ethane Formula
Ethane is only composed of carbon and hydrogen atoms, so it is classified as a hydrocarbon. It is a two-carbon containing saturated hydrocarbons consisting of only sigma bonds. The chemical formula of Ethane is and its chemical structure is shown below.
Molecular Geometry of Ethane
Ethane can be viewed as a dimer of a methyl group. The shape of the methyl group is a tetrahedral arrangement, with an angle of . When the ethane molecule is put together, the arrangement around each carbon atom is again tetrahedral with approximately bond angles.
Lewis Structure of Ethane
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. Each dot represents an electron, and a pair of dots between chemical symbols for atoms represents a bond. In there are valence electrons distributed as follows.
Skeletal Structure of Ethane
Ethane is a colourless, odourless, and flammable gas having two carbon (C) atoms and six hydrogens (H) atoms. It’s only composed of carbon and hydrogen atoms, so it is classified as a hydrocarbon. The two carbon atoms are bonded together, and three hydrogen atoms are attached to each carbon atom. All of the bonds that we see here are sigma bonds.
Three-dimensional Representation of Ethane
The three-dimensional structure of an organic compound is represented by the Wedge-dash method that has the following aspects:
Sawhorse Projection
In a sawhorse projection, the backbone carbons are represented by a diagonal line, and the terminal carbons are shown in groups, just as in the Fischer projection. A sawhorse projection can reveal staggered and eclipsed conformations in molecules.
Preparation of Ethane
Ethane is isolated on an industrial scale from natural gas and as a by-product of petroleum refining.
What is the purpose of ethane?
Production of Ethylene. One of the uses of ethane is to produce ethylene. Why is ethylene important? Ethylene is a necessary chemical in many industrial applications. For example, it is needed in the production of many commercial products we normally use, like plastic products, automotive antifreeze, and detergents.
What is ethane used for?
It is also used to produce a chemical called ethylene, which is a chemical needed in manufacturing products like plastic, automotive antifreeze, and detergent. Ethane is used as a refrigerant, which is needed in frozen food business ...
What is the chemical formula for natural gas?
Ethane is a colorless, odorless, and flammable gas with a chemical formula of C 2 H 6; it has two carbon (C) atoms and six hydrogen (H) ...
Is ethane a gas?
Ethane exists in nature as a flammable, colorless, and odorless gas. It has a chemical formula of C 2 H 6 and is a hydrocarbon. Because the chemical structure only consists of single bonds between hydrogen and carbon atoms, ethane is classified as an alkane. Ethane has many uses. It is the second most abundant component ...
Is a carbon atom a hydrocarbon?
It's only composed of carbon and hydro gen atoms, so it is classified as a hydrocarbon. Its chemical structure is shown in the following illustration. By looking at its chemical structure, the two carbon atoms are bonded together and there are three hydrogen atoms attached to each carbon atom.
Is ethane a refrigerant?
Ethane is also used as a refrigerant in very low refrigeration systems. Sometimes, it's necessary to freeze samples that need to be observed in scientific research, like cryo-electron microscopy. Cryo-electron microscopy (cryo-EM) is the use of an electron microscope to study samples at extremely low temperatures.
What is the moiety of ethane?
Related compounds may be formed by replacing a hydrogen atom with another functional group; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol, the alcohol in beverages.
What is the chemical formula for ethane?
Ethane ( / ˈɛθeɪn / or / ˈiːθeɪn /) is an organic chemical compound with chemical formula C. 2H. 6. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining.
Why is ethane favored for ethene production?
Ethane is favored for ethene production because the steam cracking of ethane is fairly selective for ethene, while the steam cracking of heavier hydrocarbons yields a product mixture poorer in ethene and richer in heavier alkenes (olefins), such as propene (propylene) and butadiene, and in aromatic hydrocarbons .
How much ethane is in natural gas?
Natural gas from different gas fields varies in ethane content from less than 1% to more than 6% by volume. Prior to the 1960s, ethane and larger molecules were typically not separated from the methane component of natural gas, but simply burnt along with the methane as a fuel.
What is the ethane barrier?
Ethane gives a classic, simple example of such a rotational barrier, sometimes called the "ethane barrier". Among the earliest experimental evidence of this barrier (see diagram at left) was obtained by modelling the entropy of ethane.
How is ethane synthesised?
In the laboratory, ethane may be conveniently synthesised by Kolbe electrolysis. In this technique, an aqueous solution of an acetate salt is electrolysed. At the anode, acetate is oxidized to produce carbon dioxide and methyl radicals, and the highly reactive methyl radicals combine to produce ethane:
What is the energy barrier of ethane?
The curve is potential energy as a function of rotational angle. Energy barrier is 12 kJ/mol or about 2.9 kcal/mol.
What is ethane made of?
Ethane is a component of natural gas. It is a hydrocarbon made of hydrogen and carbon. Ethane has 80:20 ratio of carbon and hydrogen. It is flammable and when it undergoes combustion, it leaves water and carbon dioxide. As hydrocarbons are chiefly used as fuels, it can be separated from petroleum and liquefied and can be used as fuel for automobiles. People use natural gas for cooking and other domestic purposes. Ethane finds a use for those purposes also. Ethane is used to produce ethylene by the process of steam cracking. It can be separated from methane easily under low temperatures.
Where is ethane found?
It is an organic hydrocarbon or naturally found compound of carbon and hydrogen. It is widely found under the ground in the natural gas deposit. Here it is found as a mixture of natural gas or with other components like sulphur, carbon dioxide, etc. and hydrocarbons. Underground it can be found in good quantity in shale and coal beds. It can also be isolated from natural gas. It is now being done on an industrial scale, as ethane is also used as an alternative source of energy in fuels etc. There are several countries including the USA and Saudi Arabia who have been producing it on an industrial scale.
Is ethane a natural gas?
Ethane is a natural gas which is found abundantly in nature. Being a greenhouse gas it is found in fossil fuels. In the composition of natural gas, it is found in 5 – 10% concentration.
C2H6 Compound: What is Ethane Compound?
Hydrocarbons are a class of organic chemical compound that are composed of the elements carbon and hydrogen. Ethane is one of the simplest hydrocarbons. But what is ethane? Ethane is a colorless, odorless gas at room temperature and pressure. The chemical formula is {eq}C_2H_6 {/eq} and the chemical structure is shown in the diagram.
Ethane Formula
The ethane formula is {eq}C_2H_6 {/eq} and the chemical structure is shown in the diagram above. Carbon atoms can participate in up to four chemical bonds. Hydrocarbons can have single, double or triple bonds. From the molecular formula of ethane, it consists of two carbon atoms bonded together with a single bond.
Ethane Structure
As mentioned previously, ethane structure contains only single bonds. Each carbon atom in the structure is participating in four single bonds. They are bonded to each other and to three hydrogen atoms. The structure of ethane has been investigated extensively and measured to high precision.
Ethane Uses
There are a wide variety of ethane uses in industry and research. These uses include the production of ethylene and other chemicals, as a refrigerant in cryogenic systems and in products such as anti-freeze and detergents. Ethane can also be used as fuel for power generation, either on its own or blended in natural gas.
What is the C2H6 Lewis structure?
C2H6 lewis structure: Ethane Hybridization, Molecular Geometry and shape. Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms.
What is the molecular geometry of C2H6?
Hence C2h6 has a tetrahedral molecular geometry.
What is the shape of C2H6?
However, the electron clouds that are on both the Carbon atoms will repel each other. These forces lead to the formation of a trigonal pyramidal shape for this molecule. Thus C2H6 has a trigonal pyramidal shape .
How many sigma bonds does C2H6 have?
C2H6 Hybridization. During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. Here each Carbon atom forms three sigma bonds with Hydrogen atoms and one sigma bond with a Carbon atom. As a result, four orbitals that is 1s, px, py and pz orbitals are hybridized in each Carbon atom.
How many valence electrons does carbon have?
Carbon has four valence electrons, and each Hydrogen atom has one valence electron. Total number of valence electrons in Ethane – Valence electrons of Carbon + Valence electrons of Hydrogen. = 2* 4 + 6*1 ( as there are two carbon atoms and six hydrogen atoms we will consider all of them to get the total number of valence electrons) = 14. ...
How many electrons does each carbon atom need to complete its octet?
After placing all the atoms, you might notice that each Hydrogen atom needs one electron to attain a stable structure. Similarly, Carbon needs four more electrons to complete its octet. Each Carbon atom forms bonds with three Hydrogen atoms and shares electrons.
What are the bond angles of C2H6?
C2H6 Bond Angles. The molecules with a tetrahedral molecular geometry have bond angles of 109.5 degrees, which are typically affected by lone pairs of electrons. Lone pair of electrons can change the bond angles due to their repulsive forces, but here in C2H6, as there are no lone pairs in the molecule, the bond angles in C2H6 is 109.5 degrees.