Is CaF2 a solid liquid or gas?
CsCl, NaCl and CaF2 are crystalline solids whereas glass is an amorphous solid as it does not has well developed perfectly ordered crystalline structure. Other amorphous solids include tar, rubber, plastic, butter etc. What are some non examples of a solid? Terms in this set (4) Non-metals examples : … solid.
Is CaF2 soluble or insoluble in water?
One reason is that the compound is not very soluble in water or organic solvents, has a high melting point (1,418 ??C), and in its mineral form is very stable, he explained. But when heated, CaF 2 gives rise to fluorescence, a term that historically derives its name from fluorspar.
Why is CaF2 insoluble in water?
Why CaCl2 is more soluble than CaF2? Let me give you the solubility of all the chemical mentioned… ->CaF2: is not soluble in water due to extremely high lattice energy. CaCl2 is fairly soluble in water as it’s lattice energy is lower compared to CaF2.
What is the chemical compound with the formula CaF2?
Learn about this topic in these articles:
- calcium compounds. The fluoride, CaF 2, is important to the production of hydrofluoric acid, which is made from CaF 2 by the action of sulfuric acid.
- ceramics. ...
- infrared spectroscopy. ...
What is the solubility of CaF2 in water?
The solubility of CaF2 in water at 20^oC is 15.6 mg per dm^3 of solution.
What is the solubility product of CaF2?
The solubility product of CaF2 is 1.08 × 10^-10 .
Why is CaF2 insoluble in water?
A few water molecules do not have enough strength to get in between and dissolve it. Thus, CaF2 is insoluble in water but more ionic, having high lattice energy due to small size of F−.
Is CaF2 soluble in pure water?
The solubility of CaF2 in pure water at 18^0C is 2.3 × 10^-4mol dm^-3 .
What is the solubility product of 0.1 m CaF2 solution?
The molar solubility of caf2(ksp = 5.3 1011) in 0.1 m solution of. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
How many times is solubility of CaF2 decreased?
The more soluble substance means it has a higher solubility product value. Solubility product is only applicable to the sparingly soluble solution. Hence, 100 times solubility \[Ca{{F}_{2}}\] is decreased in $4\times {{10}^{-3}}M$ KF (aq) solution as compared to pure water at${{25}^{o}}C$.
Is CaF2 aqueous?
In the situation of CaF2, Calcium is a group 2 metal and is most of the time not soluble and fluoride is not part of the soluble halogen group. So, most likely CaF2 cannot exist in an aqueous solution because neither of the elements are soluble so CaF2 will always exist as a solid inside of an aqueous solution.
Is CaF2 a salt?
0:011:25CaF2 acidic, basic, or neutral (dissolved in water)? - YouTubeYouTubeStart of suggested clipEnd of suggested clipIn this video we'll look at whether calcium fluoride that caf2 is acidic basic or neutral when it'sMoreIn this video we'll look at whether calcium fluoride that caf2 is acidic basic or neutral when it's dissolved in water the first thing we need to do is have the equation the neutralization reaction
Why is CaF2 ionic?
CaF2 is an ionic compound because, in the CaF2 molecule, calcium acts as a cation by donating its 2 extra electrons from the valence shell, and Fluoride acts as an anion by accepting the electrons from Calcium, completing its octet. Therefore, Calcium and fluoride together form an ionic bond, i.e., CaF2.
Is CaF2 soluble in HCL?
For instance, when the hydrochloric acid concentration in a reaction solution is 3%, the solubility of calcium fluoride can be increased to about 0.2%, and when the hydrochloric acid concentration in the reaction solution is 6%, the solubility of calcium fluoride to about 0.4%.
How do you find molar solubility?
0:549:00How to find molar solubility and Ksp - Real Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clip2 plus n o h minus. And notice since there's the 2 in from my calcium that h minus is gonna getMore2 plus n o h minus. And notice since there's the 2 in from my calcium that h minus is gonna get squared. So that's my KSP.
Is cacl2 soluble in water?
WaterAcetic acidAcetoneCalcium chloride/Soluble in
How to measure maternal fluoride levels?
Gas chromatography was used to measure the maternal and fetal plasma inorganic fluoride values at term in 91 women. They were assigned to one of four groups: group A were untreated controls; group B received a single daily dose of 1.5 mg of fluoride (as calcium fluoride) during the final trimester of pregnancy; group C was given a single dose of 1.5 mg of fluoride (as sodium fluoride) and group D was given 2 daily doses of fluoride (as sodium fluoride) totaling 1.5 mg. There was a significant difference between the cord plasma fluoride levels of the newborns in the untreated group (mean: 27.8 ug/L) and that of the combined supplemented groups B, C and D (mean: 58.3 ug/L). There was no difference between the average fluoride levels in the three supplemented groups. In 17 women, gestation time between 19 and 34 weeks, fluoride levels were measured in the mother (mean: 17.11 ug/L) and in the foetus by cord tapping (mean: 35.64 ug/L). In 4 other mothers, was given a dose of 1 mg of sodium fluoride; one hour later, mean cord fluoride level was 86.5 ug/L. These results indicate that placental transfer of fluoride occurs early during pregnancy and that supplementation during the final trimester of pregnancy will significantly elevate cord plasma fluoride concentrations.
What is ferrous fluorspar used for?
In ferrous metallurgy it is used as a flux to incr fluidity of the slag. ... Synthetic fluorspar is used in the optical industry (transmits UV rays), and pure calcium fluoride used as a catalyst in dehydration and dehydrogenations. Used to fluoridate drinking water. Budavari, S. (ed.).
Is fluorite mine dust pathogenic?
The pathogenicity of mixed dust from a fluorite mine was studied by animal experiments and in vitro tests. Animal experiments showed that calcium fluorite can induce only a foreign body reaction in the lungs; the fibrous nodular lesions induced by the fluorite mine dust are due mainly to its silica component. It was demonstrated that either silica or the mixed dust of a fluorite mine can stimulate pulmonary alveolar macrophages to release fibrogenetic factors in vitro, but calcium fluorite cannot. It was also demonstrated that having engulfed calcium fluorite, silica, or fluorite mine mixed dust, pulmonary alveolar macrophages release an elastase-active substance. It was suggested that the emphysematous lesion seen in autopsy material of pneumoconiosis of fluorite mine workers may be caused by calcium fluorite and silica.
Is calcium fluoride safe during pregnancy?
EMBRYOTOXIC STUDIES OF CALCIUM FLUORIDE IN MICE OVER SEVERAL GENERATIONS INDICATED THAT ADMIN DURING PREGNANCY WAS RELATIVELY SAFE COMPARED TO OTHER FLUORIDES. ACUTE (IP) & CHRONIC (ORAL) LD50 VALUES ARE GIVEN.
Is 20 °C evaporation harmful?
Evaporation at 20 °C is negligible; a harmful concentration of airborne particles can, however, be reached quickly on spraying.
Is calcium fluoride soluble in water?
CALCIUM FLUORIDE has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water -reactive.
class 5
The Fish Tale Across the Wall Tenths and HundredthsParts and Whole Can you see the Pattern?
class 9
Circles Coordinate Geometry What is Democracy? Why Democracy?Nazism and the Rise of Hitler Socialism in Europe and the Russian Revolution
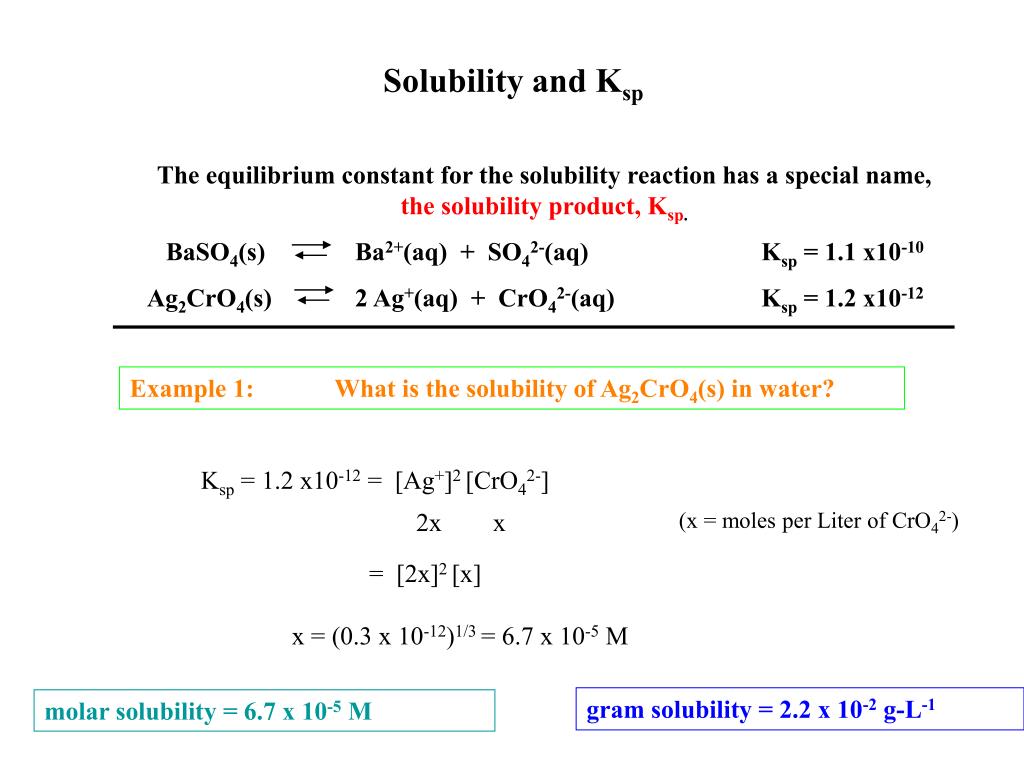
What is the solubility product constant?
The solubility product constant, Ksp, essentially tells you how far to the left this equilibrium lies. In your case, calcium fluoride, CaF2, is considered insoluble in aqueous solution. The small amounts of calcium fluoride that do dissociate will produce calcium cations, Ca2+, and fluoride anions, F−, in solution. CaF2(s) ⇌ Ca2+ (aq) +2F− (aq)
What does the molar solubility of an insoluble ionic compound tell you?
The molar solubility of an insoluble ionic compound tells you how many moles of said compound you can dissolve in one liter of water. Insoluble ionic compounds do not dissociate completely in aqueous solution, which implies that an equilibrium is established between the undissolved solid and the dissolved ions.