Where are ribosomal subunits synthesized in eukaryotes?
In eukaryotes, the process takes place both in the cell cytoplasm and in the nucleolus, which is a region within the cell nucleus. Subsequently, question is, where are ribosomal subunits synthesized? The ribosomalproteins are synthesizedin the cytoplasm and then transferred to the nucleolus for assembly into subunits.
Is ribosome assembly eukaryotic or prokaryotic?
Eukaryotic ribosome assembly is best understood in yeast where a large number of ribosome precursors have been isolated and their components identified. The non-ribosomal proteins associated with pre-ribosomal particles are for a large majority of them essential for ribosome biogenesis.
Is Assembly of ribosomal subunits self-assembly?
From in vitro reconstitution assays made with bacterial systems, we learned that assembly of the ribosomal subunits from the individual proteins and rRNA components is a self-assembly process that only requires a heating step (reviewed in Nierhaus, 1991 ).
Where does the Assembly of ribosomes begin?
Where does the assembly of ribosomes begin? The ribosome is made of ribosomal RNA (rRNA)and protein. Eukaryote ribosomes are produced and assembled in the nucleolus within the nucleus and then exported to the cytoplasm. Ribosomes 'read' mRNA and translate the nucleotide code to protein.
What is the site for ribosomal subunit assembly?
The most prominent substructure within the nucleus is the nucleolus (see Figure 8.1), which is the site of rRNA transcription and processing, and of ribosome assembly.
Where are the ribosomal subunits assembled in eukaryotes quizlet?
1) Subunits of ribosomes are assembled in the nucleolus and pass through the nuclear membrane via the nuclear pores.
Which is the site of initial ribosomal assembly?
the nucleolusThe ribosome synthesis pathway in eukaryotes. The initial stages of ribosome synthesis take place in the nucleolus.
What are the 3 site in large ribosomal subunit?
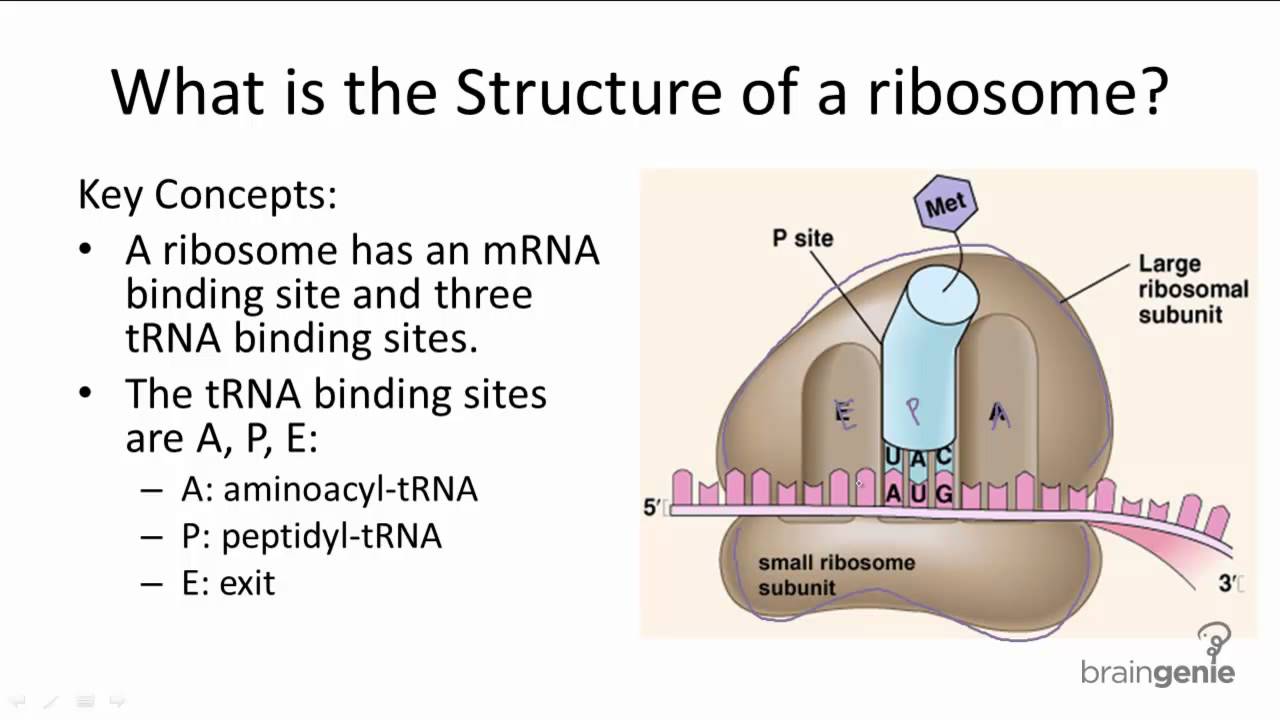
The large ribosomal subunit has three places that can bind tRNA: the A site, the P site, and the E site.
Where within a eukaryotic cell is ribosomal RNA rRNA synthesized?
the nucleolusMolecules of rRNA are synthesized in a specialized region of the cell nucleus called the nucleolus, which appears as a dense area within the nucleus and contains the genes that encode rRNA.
In which cellular location in a eukaryote do rRNA synthesis and ribosome assembly occur?
The nucleolus synthesizes ribosomal RNA (rRNA). (ribosomes made in the nucleus, but ribosomes synthesize proteins in cytoplasm).
Where precisely are ribosomes assembled in eukaryotic cells?
the nucleolusThis darkly staining region is called the nucleolus, and it's the site in which new ribosomes are assembled. Diagram of the parts of the nucleus of a eukaryotic cell.
What is the site of assembly of 80S ribosomes?
cytoplasmThe general consensus is that 80S, which correspond to ribosomes at the initiation, elongation, termination, or post-termination stages of translation, are present only in the cytoplasm in eukaryotes (Jackson et al.
What is the site of synthesis of rRNA and assembly of rRNA and proteins into ribosomal subunits?
Nucleolus NucleoliNucleoli are a prominent feature of an interphase nucleus (seeFig. 1.2). They are the site of most of the synthesis of ribosomal RNA (rRNA) and assembly of ribosome subunits.
What is a site P site and e site?
The A site accepts an incoming tRNA bound to an amino acid. The P site holds a tRNA that carries a growing polypeptide (the first amino acid added is methionine (Met)). The E site is where a tRNA goes after it is empty, meaning that it has transferred its polypeptide to another tRNA (which now occupies the P site).
What is the E site of a ribosome?
The E-site is the third and final binding site for t-RNA in the ribosome during translation, a part of protein synthesis. The "E" stands for exit, and is accompanied by the P-site (for peptidyl) which is the second binding site, and the A-site (aminoacyl), which is the first binding site.
What are the 3 sites on a ribosome?
Three tRNA-binding sites are located on the ribosome, termed the A, P and E sites.
Where does ribosome synthesis take place?
The ribosome synthesis pathway in eukaryotes. The initial stages of ribosome synthesis take place in the nucleolus. The first step is the association of newly synthesized 35S rRNA with 40S processing proteins and 40S ribosomal proteins which form a complex with the future 18S rRNA sequence even before the transcript is completed (the co-transcriptional assembly stage). After completion of rRNA transcription, the 35S rRNA and its associated proteins form a 90S pre-ribosome particle which contains numerous 40S processing factors and 40S ribosomal proteins but very few 60S processing proteins or 60S ribosomal proteins. RNA cleavage releases U3 snoRNP and separates the 90S particle into 40S and 60S pre-ribosome particles. The latter recruits 60S processing proteins and 60S ribosomal proteins, and the separate pre-ribosome complexes are exported out of the nucleolus into the nucleus and cytoplasm. Most of our knowledge about this pathway has been compiled from studies in budding yeast; see text for further details.
Why are ribosomes coordinated?
Because of the extremely high energy cost of ribosome synthesis for the cell, the various activities are coordinated spatio-temporally for efficiency. A recent further proof of such coordination is the finding that rRNA transcription and rRNA processing are coordinated through a subset of proteins shared by the two processes [ 14 ]. In addition, a recent electron microscopy study has shown that 40S-subunit processing proteins associate with and compact the rRNA within seconds of completion of rRNA transcription [ 15 ]. These findings confirm the existence of a fine-tuned molecular assembly line where tasks are performed sequentially and without intervening delays.
What have we learned about ribosomes?
In conclusion, we have learned a great deal about ribosomes in this decade, in large part because of high-resolution crystal structures that revealed the molecular details of peptide-bond formation and the RNA-driven nature of the ribosome's catalytic activity [ 36 ]. Almost three decades after the first identification of 90S pre-ribosomes, these particles have been purified and characterized [ 19, 21 ]. Subsequently, the compilation of around 200 trans -acting proteins involved in ribosome synthesis has prompted numerous genetic and biochemical studies aimed at their characterization (see [ 6, 7, 31] for reviews). Remarkably, most of these auxiliary proteins are essential in budding yeast, indicating relatively low tolerance of cells for incomplete or defective ribosomes. Furthermore, the majority of trans -acting proteins are conserved from yeast to humans, strongly suggesting that the overall pathway of ribosome synthesis is conserved among eukaryotes. The future challenge will be to decipher the exact cellular functions of all trans -acting proteins. One way to realize this goal is through the combination of computational predictions and experiments [ 23, 34 ]. Ultimately, molecular details of their functions will be deduced from structural studies [ 15, 37 ].
What is the pre-ribosome of 35S RNA?
After completion of rRNA transcription, the 35S rRNA and its associated proteins form a 90S pre-ribosome particle which contains numerous 40S processing factors and 40S ribosomal proteins but very few 60S processing proteins or 60S ribosomal proteins. RNA cleavage releases U3 snoRNP and separates the 90S particle into 40S ...
What is the 90S pre-ribosome?
Classic work in the early 1970s identified a large 90S pre-ribosome, which is eventually converted into the precursors of the 40S and 60S subunits. It was also shown that disruptions of either the large or small subunit synthesis pathway do not necessarily impact on the cytoplasmic export of the unaffected subunit [ 3 ]. Until 2001, however, most of our knowledge about auxiliary ribosome synthesis factors was based on genetic studies and biochemical experiments, providing what turned out to be a limited picture of the ribosome maturation pathway (for reviews see [ 2, 3 ]).
How many subunits are there in ribosomes?
Eukaryotic ribosomes have two unequal subunits, designated small subunit (40S) and large subunit (60S) according to their sedimentation coefficients. Both subunits contain dozens of ribosomal proteins arranged on a scaffold composed of ribosomal RNA (rRNA). The small subunit monitors the complementarity between tRNA anticodon and mRNA, ...
What is the ribosome?
The ribosome selects aminoacylated transfer RNAs (tRNAs) based on the sequence of a protein-encoding messenger RNA (mRNA) and covalently links the amino acids into a polypeptide chain. Ribosomes from all organisms share a highly conserved catalytic center.
Which ribosome is responsible for signaling?
Ribosomal proteins with roles in signaling. Two 40S ribosomal proteins ( RACK1 and RPS6 (or eS6)) have been implicated in cellular signaling: RACK1, first described as the receptor of activated protein kinase C (PKC), is an integral component of the eukaryotic ribosome and is located at the back of the head.
Where are ribosomes produced?
The ribosome units leave the nucleus through the nuclear pores and unite once in the cytoplasm for the purpose of protein synthesis.
How do newly synthesized proteins exert their functions in the cell?
To exert their functions in the cell newly synthesized proteins must be targeted to the appropriate location in the cell, which is achieved by protein targeting and translocation systems. The growing polypeptide leaves the ribosome through a narrow tunnel in the large subunit. The region around the exit tunnel of the 60S subunit is very similar to the bacterial and archaeal 50S subunits. Additional elements are restricted to the second tier of proteins around the tunnel exit, possibly by conserved interactions with components of the translocation machinery. The targeting and translocation machinery is much more complex in eukaryotes.
What are the PDB identifiers for ribosomes?
Proteins shared only between eukaryotes and archaea are shown in orange, and proteins specific to eukaryotes are shown in red. PDB identifiers 4a17, 4A19, 2XZM aligned to 3U5B, 3U5C, 3U5D, 3U5E. Ribosomes are a large and complex molecular machine that catalyzes the synthesis of proteins, referred to as translation.
What is the 40S subunit of the ribosome?
Eukaryotic ribosome. The 40S subunit is on the left, the 60S subunit on the right. The ribosomal RNA ( rRNA) core is represented as a grey tube, expansion segments are shown in red. Universally conserved proteins are shown in blue. These proteins have homologs in eukaryotes, archaea and bacteria. Proteins shared only between eukaryotes ...
What are the binding sites of ribosomes?
The binding sites for the aminoacyl-transfer RNA (tRNA) (A site), peptidyl-tRNA (P site), and deacylated tRNA (exit or E site) on the bacterial ribosome are composed predominantly of rRNA (Yusupov et al. 2001; Selmer et al. 2006). This rRNA is conserved in archaeal and eukaryotic ribosomes, suggesting that the basic mechanism by which the ribosome distinguishes the cognate tRNA from the near- or noncognate tRNAs at the A site during decoding (Ogle and Ramakrishnan 2005; Schmeing et al. 2011) is also likely to be conserved. Nevertheless, many r-proteins encroach on the tRNA-binding sites and appear to play important roles in decoding, accommodation, and stabilization of tRNAs (Fig. 3C) (Yusupov et al. 2001; Selmer et al. 2006; Jenner et al. 2010b). These r-proteins may be responsible for the slightly different positioning of tRNAs on the eukaryotic ribosome compared with the bacterial ribosome (Budkevich et al. 2011). On the SSU a conserved loop of S12 participates in monitoring of the second and third positions of the mRNA–tRNA codon–anticodon duplex (Ogle and Ramakrishnan 2005). Additionally, the carboxy-terminal extensions of r-proteins S19 and S9/S13 stretch from globular domains located on the head of the SSU to interact with anticodon stem-loop (ASL) regions of A- and P-tRNA, respectively, whereas S7, and to a lesser extent S11, interacts with the ASL of E-tRNA (Fig. 3C) (Yusupov et al. 2001; Selmer et al. 2006; Jenner et al. 2010b). Although these tRNA interactions are likely to be maintained in eukaryotic 80S ribosomes, additional interactions are probable on the SSU because of the presence of extensions of four eukaryotic r-proteins that approach the tRNA-binding sites, namely, the amino-terminal extensions of S30e and S31e that reach into the A site; S25e, which is positioned between the P and E sites; and S1e at the E site (Fig. 3C) (Armache et al. 2010b; Ben-Shem et al. 2011; Rabl et al. 2011). S31e is expressed with an amino-terminal ubiquitin fusion, suggesting that the lethality from lack of cleavage (Lacombe et al. 2009) arises because of the inability of tRNA and/or initiation factors to bind to the SSU (Rabl et al. 2011).
What are ribosomes made of?
All ribosomes are composed of two subunits, both of which are built from RNA and protein (Figs. (Figs.11and and2).2). Bacterial ribosomes, for example of Escherichia coli, contain a small subunit (SSU) composed of one 16S ribosomal RNA (rRNA) and 21 ribosomal proteins (r-proteins) (Figs. (Figs.1A1A and and1B)1B) and a large subunit (LSU) containing 5S and 23S rRNAs and 33 r-proteins (Fig. 2A). Crystal structures of prokaryotic ribosomal particles, namely, the Thermus thermophilusSSU (Schluenzen et al. 2000; Wimberly et al. 2000), Haloarcula marismortuiand Deinococcus radioduransLSU (Ban et al. 2000; Harms et al. 2001), and E. coliand T. thermophilus70S ribosomes (Yusupov et al. 2001; Schuwirth et al. 2005; Selmer et al. 2006), reveal the complex architecture that derives from the network of interactions connecting the individual r-proteins with each other and with the rRNAs (Brodersen et al. 2002; Klein et al. 2004). The 16S rRNA can be divided into four domains, which together with the r-proteins constitute the structural landmarks of the SSU (Wimberly et al. 2000) (Fig. 1A): The 5′ and 3′ minor (h44) domains with proteins S4, S5, S12, S16, S17, and S20 constitute the body (and spur or foot) of the SSU; the 3′ major domain forms the head, which is protein rich, containing S2, S3, S7, S9, S10, S13, S14, and S19; whereas the central domain makes up the platform by interacting with proteins S1, S6, S8, S11, S15, and S18 (Fig. 1B). The rRNA of the LSU can be divided into seven domains (including the 5S rRNA as domain VII), which—in contrast to the SSU—are intricately interwoven with the r-proteins as well as each other (Ban et al. 2000; Brodersen et al. 2002) (Fig. 2A). Structural landmarks on the LSU include the central protuberance (CP) and the flexible L1 and L7/L12 stalks (Fig. 2A).
What is the protein to RNA ratio of a bacterial SSU?
The protein-to-RNA ratio of bacterial SSU is ∼1:2 , whereas the dramatic increase in r-protein mass for the eukaryotic SSU results in an almost 1:1 ratio. The SSU structures reveal that most of the additional eukaryotic-specific r-proteins and extensions cover the back of the SSU particle, forming a web of interactions with each other as well as with conserved r-proteins and rRNA (Fig. 1C–E) (Ben-Shem et al. 2011; Rabl et al. 2011). The beak of the eukaryotic SSU has acquired three r-proteins, S10e, S12e, and S31e, which appear to compensate for the reduced h33 compared with the bacterial SSU rRNA (Rabl et al. 2011). R-proteins are also seen to interact with the expansion segments ES3Sand ES6S, via r-proteins S4e, S6e, S7e, and S8e (Fig. 3B). S6e has a long carboxy-terminal helix that stretches from the left to right foot, and that is phosphorylated in most eukaryotes (Meyuhas 2008). Based on the peripheral position of S6e, any regulation of translation via S6e phosphorylation is likely to be via indirect recruitment of specific regulatory factors (Rabl et al. 2011). The mRNA exit site on the eukaryotic SSU also differs from the bacterial one because of the presence of S26e and S28e surrounding the 3′ end of the 18S rRNA (Fig. 3F) (Armache et al. 2010a; Rabl et al. 2011). S26e overlaps the binding position of the E. colir-protein S21p (Schuwirth et al. 2005), whereas S28e has a similar fold to the bacterial RNA-binding domain of r-protein S1p (Rabl et al. 2011). Such differences may reflect the distinct elements found in the 5′ untranslated regions of eukaryotic mRNAs, as well as the divergence in the translation initiation phase from bacteria (Sonenberg and Hinnebusch 2009). Indeed, eIF3, which is absent in bacteria, interacts with this general region of the SSU (Bommer et al. 1991; Srivastava et al. 1992; Siridechadilok et al. 2005), as do internal ribosome entry site (IRES) elements present in the 5′ untranslated region of viral mRNAs (Spahn et al. 2001b; Schuler et al. 2006; Muhs et al. 2011). S30e replaces part of S4 at the mRNA entry site of the eukaryotic SSU and has conserved lysine residues that extend into the mRNA channel (Fig. 3G), suggesting that S30e, together with S3, plays a role in unwinding mRNA secondary structure (Rabl et al. 2011). S3 has a long carboxy-terminal extension that spans across S17e and interacts with RACK1 (Fig. 3G) (Rabl et al. 2011). RACK1 is a scaffold protein that binds to several signaling proteins, therefore connecting signaling transduction pathways with translation (Nilsson et al. 2004). Thus, in addition to stabilization of rRNA ES architecture of the ribosome, eukaryotic-specific r-proteins and extensions appear to be important for binding of eukaryotic-specific regulatory factors, particularly factors that interact with the SSU to regulate translation initiation of specific mRNAs.
What are the changes in the ribosome?
These changes involve intersubunit rotation, as well as swiveling of the head domain of the SSU (Fig. 5A). The interactions between the ribosomal subunits, or “bridges,” change with each of these rearrangements, and are therefore dynamic in composition. The intersubunit bridges were originally mapped in bacteria by modeling high-resolution SSU and LSU structures into cryo-EM reconstructions and low-resolution X-ray crystal structures (Gabashvili et al. 2000; Yusupov et al. 2001; Valle et al. 2003), and in more recent high-resolution structures of the intact bacterial ribosome (Schuwirth et al. 2005; Dunkle et al. 2011). The bridges in eukaryotic ribosomes have been mapped using similar approaches. The high-resolution structures of the yeast 80S ribosome now provide an atomic-resolution view of the bridges for rotated states of the ribosome (Ben-Shem et al. 2011), and cryo-EM reconstructions of translating ribosomes at ∼5- to 6-Å resolution reveal the intersubunit bridges in the unrotated state of the ribosome (Armache et al. 2010a,b).
How many r-proteins are in the 80S ribosome?
The yeast 80S ribosome contains 79 r-proteins (SSU, 33; LSU, 46), 35 of which (SSU, 15; LSU, 20) have bacterial/archaeal homologs, whereas 32 (SSU, 12; LSU, 20) have only archaeal homologs (Lecompte et al. 2002). Thus, 12 (SSU, 6; LSU, 6) r-proteins of the yeast 80S are specific for eukaryotes. Cytoplasmic 80S ribosomes of Tetrahymenaand higher eukaryotes, such as humans, contain an additional LSU r-protein, L28e, and thus have 13 eukaryotic-specific r-proteins and 80 (SSU, 33; LSU, 47) in total. Together with the ES, the additional r-proteins/r-protein extensions form an intricate layer of additional RNA–protein mass that locates predominantly to the solvent surfaces of the ribosome (Figs. (Figs.1C1C and and2B).2B). More than half of the conserved r-proteins contain extensions, which in some cases, such as S5, L4, L7, and L30, establish long-distance interactions far (50–100 Å) from the globular core of the protein. Interaction of eukaryotic-specific extensions with conserved core proteins using interprotein shared β-sheets has been noted, for example, between L14e and L6 (Ben-Shem et al. 2011) as well as L21e and L30 (Klinge et al. 2011).
What is the role of the ribosomal tunnel?
However, growing evidence indicates that the tunnel plays a more active role in regulating the rate of translation, in providing an environment for early protein folding events, and in recruiting translation factors to the tunnel exit site (Wilson and Beckmann 2011). At the simplest level, long stretches of positively charged residues, such as arginine or lysine, in an NC can reduce or halt translation, most likely through interaction with the negatively charged rRNA in the tunnel (Lu and Deutsch 2008). More specific regulatory systems also exist in bacteria and eukaryotes, in which stalling during translation of upstream open reading frames (uORFs of the cytomegalovirus [CMV] gp48 and arginine attenuator peptide [AAP] CPA1 genes) or leader peptides (TnaC, SecM) leads to modulation of expression of downstream genes (Tenson and Ehrenberg 2002). Interestingly, the translational stalling events depend critically on the sequence of the NC and the interaction of the NC with the ribosomal tunnel. Cryo-EM reconstructions of bacterial TnaC- and SecM-stalled 70S ribosomes (Seidelt et al. 2009; Bhushan et al. 2011) and eukaryotic CMV- and AAP-stalled 80S ribosomes (Bhushan et al. 2010b) reveal the distinct pathways and conformations of the NCs in the tunnel as well as the interactions between the NCs and tunnel wall components. Compared with bacteria, eukaryotic r-protein L4 has an insertion that establishes additional contacts with the CMV- and AAP-NCs (Bhushan et al. 2010b), whereas the bacterial stalling sequences interact predominantly with L22 (Seidelt et al. 2009; Bhushan et al. 2011). The dimensions of the ribosomal tunnel preclude the folding of domains as large as an IgG domain (∼17 kDa) (Voss et al. 2006), whereas α-helix formation has been demonstrated biochemically (Deutsch 2003; Woolhead et al. 2004) and visualized structurally within distinct regions of the tunnel (Bhushan et al. 2010a). Folding of NCs within the tunnel may have implications for not only protein folding, but also downstream events, such as recruitment of chaperones or targeting machinery (Bornemann et al. 2008; Berndt et al. 2009; Pool 2009).
What proteins are involved in tRNA binding?
Additional stabilization of tRNA binding is observed via interaction between LSU r-proteins with the elbow regions of tRNAs, namely, the A- and P-tRNA, through contact with conserved r-proteins L16 and L5, respectively, as well as the E-tRNA with the L1 stalk (Yusupov et al. 2001; Selmer et al. 2006; Jenner et al. 2010b). The carboxyl terminus of the bacterial-specific r-protein L25p also interacts with the elbow region of A-tRNA (Jenner et al. 2010b). This r-protein is absent in archaeal and eukaryotic ribosomes. At the peptidyltransferase center (PTC) of the LSU, the CCA ends of the A- and P-tRNAs are stabilized through interaction with the conserved A- and P-loops of the 23S rRNA, thus positioning the α-amino group of the A-tRNA for nucleophilic attack on the carbonyl carbon of the peptidyl-tRNA (Leung et al. 2011). The high sequence and structural conservation of the PTC and of the tRNA substrates suggests that the insights into the mechanism of peptide bond formation gained from studying archaeal and bacterial ribosomes (Simonovic and Steitz 2009) are transferable to eukaryotic ribosomes. Nevertheless, the varying specificity for binding of antibiotics to the PTC of bacterial versus eukaryotic LSU indicates that subtle differences do in fact exist (Wilson 2011). In addition to differences in the conformation of rRNA nucleotides, one of the major differences between the bacterial and eukaryotic PTC is related to r-proteins. Eukaryotic L16 contains a highly conserved loop that reaches into the PTC and contacts the CCA end of the P-tRNA (Fig. 3D) (Armache et al. 2010b; Bhushan et al. 2010b). This loop is absent in bacteria, and instead the space is occupied by the amino-terminal extension of bacterial-specific r-protein L27p (Fig. 3E) (Voorhees et al. 2009). The binding site of the CCA end of the E-tRNA on the eukaryotic LSU resembles the archaeal, rather than the bacterial, context. Whereas bacterial-specific r-protein L28p contributes to the E site of the bacterial LSU (Selmer et al. 2006), the archaeal and eukaryotic r-protein L44e contains an internal loop region (Fig. 2D) through which the CCA end of the E-tRNA inserts (Schmeing et al. 2003). Moreover, the carboxyl terminus of L44e is longer in eukaryotes, such as yeast, than in archaea, providing the potential for additional interactions with the P- and/or E-tRNA. Nevertheless, the E site restricts binding of only deacylated tRNAs via a direct interaction between the 2′OH of A76 and the base of C2394 (E. coli23S rRNA numbering) (Schmeing et al. 2003; Selmer et al. 2006). The base equivalent to C2394 is conserved across all kingdoms (Cannone et al. 2002), suggesting a universal mechanism of deacylated-tRNA discrimination at the E site on the LSU.
Where does ribosome synthesis occur?
Ribosome synthesis is a highly complex and coordinated process that occurs not only in the nucleolus but also in the nucleoplasm and the cytoplasm of eukaryotic cells. Based on the protein composition of several ribosomal subunit precursors recently characterized in yeast, a total of more than 170 factors are predicted to participate in ribosome biogenesis and the list is still growing. So far the majority of ribosomal factors have been implicated in RNA maturation (nucleotide modification and processing). Recent advances gave insight into the process of ribosome export and assembly. Proteomic approaches have provided the first indications for a ribosome assembly pathway in eukaryotes and confirmed the dynamic character of the whole process.
How are ribosomes formed in yeast?
The formation of functional ribosomes in yeast starts with the synthesis of pre-rRNAs by the activity of the RNA polymerase I. The first detectable intermediate (35S in yeast) contains 5′ and 3′ external transcribed region (ETS) as well as the mature 18S rRNA, 5.8S and 25S rRNA interspersed with non coding sequences ITS1 and ITS2. The 18S rRNA will be the rRNA component of the small 40S subunit whereas the 5.8S and 25S rRNA together with the 5S rRNA, synthesized independently by RNA polymerase III, will constitute the RNA component of the 60S subunit.
What are the RNAs that are used in eukaryotic RNA modification?
In contrast to bacterial systems where formation of 2′-O-methyl groups and pseudouridines implies specific enzymes, the equivalent rRNA modifications in eukaryotes are directed by small nucleolar RNAs, the box C/D for methylations and H/ACA snoRNAs for pseudouridylations, respectively. These RNAs are found in small RNPs in complex with specific integral proteins: fibrillarin-Nop1, Nop56, Nop58/Nop5, Snu13 for boxC/D snoRNPs and Cbf5, Gar1, Nhp2 and Nop10 for the H/ACA snoRNPs ( Tollervey and Kiss, 1997, Kiss, 2001 and references therein). The mechanism of site selection has been elucidated and depends on the complementarity of short conserved regions of the snoRNAs with rRNA sequences in the region of the modified nucleotide ( Balakin et al., 1996, Cavaille et al., 1996, Kiss-Laszlo et al., 1996 ). It appears that a single enzyme is responsible for each type of modification, the putative 2′-O-methyltransferase, fibrillarin/Nop1, ( Tollervey et al., 1993) and the pseudouridine synthase, Cbf5, ( Lafontaine et al., 1998a ). The advantage of snoRNA guides is that modification can take place before completion of the RNA transcript, thus facilitating the folding of the pre-RNA precursor. Whereas the activity of the snoRNAs seems not to be regulated, their biosynthesis is coordinated with that of components of the translational machinery ( Bachellerie et al., 2000 ), either because the vast majority of vertebrate and some of yeast guide RNAs are hosted by intronic sequences of ribosomal proteins or because the promoter regions of mono- or polycistronic snoRNA genes contain the binding site for Rap1 known to control the transcriptional activity of yeast ribosomal proteins ( Qu et al., 1999 ).
How do ribosomes interact with RNA?
Primary binding proteins (i.e. S8, S15) are typically globular proteins that often bind to three- or four-helix junctions and tie together distant parts of the RNA which initiates the correct tertiary fold of RNA. Subsequent binding of secondary and tertiary binders will accelerate the formation of tertiary contacts and stabilize the final RNA conformation. A detailed chronological order of events for the assembly of the central domain of 30S subunits has emerged from data combining biochemical, biophysical and crystallographic approaches ( Agalarov et al., 2000, Agalarov and Williamson, 2000) that constitute guidelines for a hierarchical assembly pathway of the assembling ribosome.
What are the three substructures of the nucleolus?
The organization of the nucleolus into three substructures, the fibrillar centers (FCs), the dense fibrillar compartment (DFC) and the granular component (GC) is now well established for lower and higher eukaryotes. By sequestering specific ribosome processing factors, these ultrastructures are believed to participate in the spatio-temporal ordering of the ribosome synthesis pathway ( Scheer and Hock, 1999 ). The analysis of the DFC revealed that it contains fibrillarin (Nop1p in yeast), snoRNAs (U3 for example) and rRNA ( Beven et al., 1996) and that this particular region of the nucleolus coincides with the site of RNA polymerase I transcription ( Cmarko et al., 2000 ). Altogether, this locates the initial 90S preribosomes within the DFC region.
How many factors are involved in pre-ribosomal complexes?
Recently, the identification of protein complexes associated with more than 50 different factors generated an exhaustive inventory of pre-ribosomal proteins (see Table 1). Starting with a list of known pre-ribosomal factors, we extracted the corresponding interaction map from the available protein composition of the preribosomal complexes ( Bassler et al., 2001, Harnpicharnchai et al., 2001, Dragon et al., 2002, Gavin et al., 2002, Grandi et al., 2002, Nissan et al., 2002, Fatica et al., 2002a) to generate clusters of pre-ribosomal proteins ( Fig. 2). Visual inspection of the resulting groups of proteins, the presence of known factors in each group and the available data on the co-purified pre-rRNAs for a subset of these proteins, allowed us to assign the different groups to 90S, pre-60S, pre-40S or late pre-60S complexes.
What are the most common modified nucleotides in RNA?
The isomerization of uridines to pseudouridines ( Ψ s) and methylation of 2′-hydroxyl of riboses are the most prevalent modified nucleotides in rRNAs. About 100 rRNA sites of each type are modified in human ( Maden, 1990 ). This number decreases to 50 in the yeast Saccharomyces cerevisiae and E. coli ribosomes contain only four ribose-methylated nucleotides and ten pseudouridines in addition to ten base methylations at various positions ( Rozenski et al., 1999 ). Two main features are associated with modified nucleotides: (i) none of them seems important individually but globally they are believed to play a general role in RNA conformation and stabilization, (ii) they tend to be concentrated in functional rRNA regions and to fine-tune ribosome activity in translation ( Kowalak et al., 1995, Green and Noller, 1996, Nissen et al., 2000 ). This clustering has been confirmed by placing the known rRNA modification positions on a three dimensional map for E. coli and yeast ribosomes ( Decatur and Fournier, 2002 ). More generally, the rRNA modifications act as a molecular mortar to stabilize the required RNA conformations ( Ofengand, 2002) and are absent from the regions where the ribosomal proteins bind ( Decatur and Fournier, 2002 ). Knowledge of how modifications influence RNA conformation and dynamics has come from the study of the smaller tRNA molecule ( Agris, 1996 ). This study also established a clear relationship between modified nucleosides, tight Mg 2+ binding and the resulting RNA conformation.