What is the relationship between molarity and density?
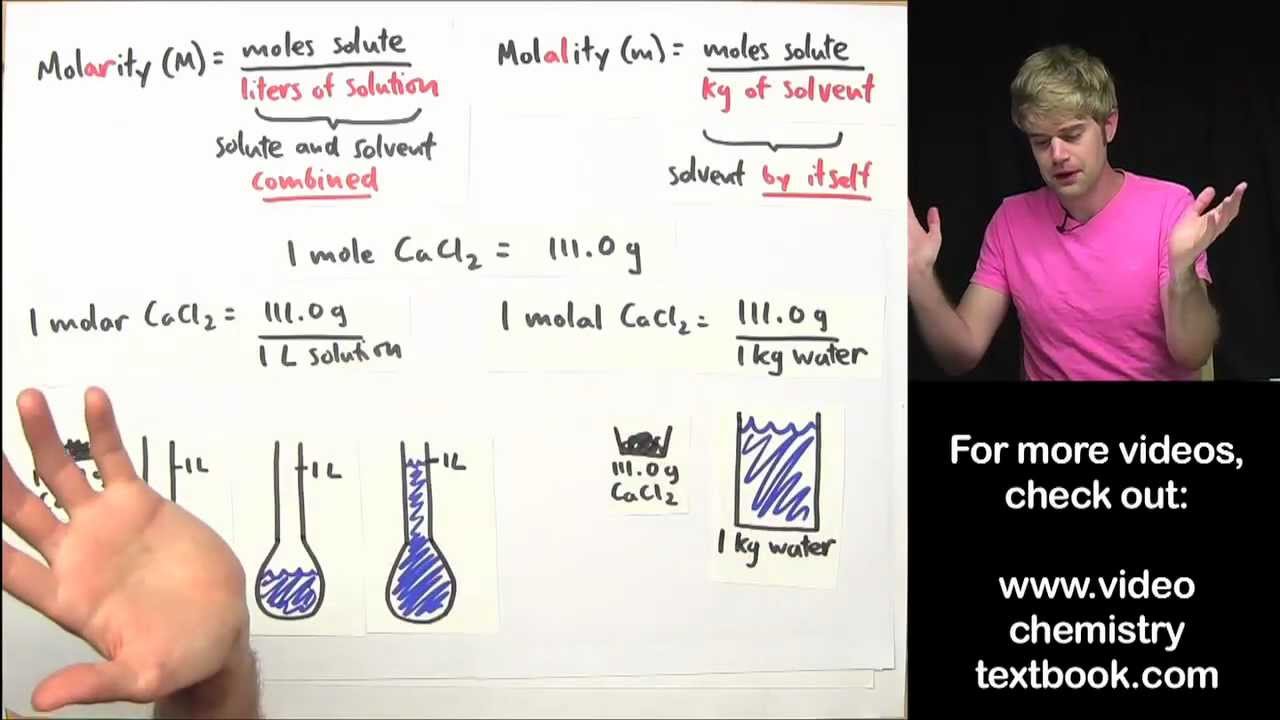
Relation between molarity and molality in terms of density and molar mass: M=md/[1+(mW/1000)] Here M = molarity. m= molality. W= molar mass of solute. d= density in g/cm³
How to calculate molarity using density?
Molarity using Molality Solution
- Convert Input (s) to Base Unit
- Evaluate Formula
- Convert Result to Output's Unit
How does molarity affect density?
Molarity is a measure of the concentration of a solute in a solvent. As a general rule, the more of a substance that is dissolved in a fixed volume of solvent (in other words, the greater the molarity of a solution) the greater its density will become. # difference between molarity and density.
How do you find molarity from density?
Molarity = (percentage concentration * density ) / (molar mass * 100) the units required for this calculation are: Knowing the density of the acid to be 1.413 g/ml, we can calculate the weight of 1 l of 70% hno 3 to be 1413 Mols of water = (100 g) / (18.015 g/mol) = 5.55 mol.
What is the relation between molarity and molality in terms of density?
Molality depends only on the mass of the solvent, but molarity is dependent on the volume of solution, and volume can be changed through a change in pressure and temperature. Also, therefore unlike molality, molarity is dependent on pressure.
Does molarity depend on density?
Although molarity is commonly used to express concentrations for reactions in solution or for titrations, it does have one drawback—molarity is the number of moles of solute divided by the volume of the solution, and the volume of a solution depends on its density, which is a function of temperature.25-Apr-2020
How does density relate to molality?
The unit of molality is, therefore, expressed in moles per kilogram. The formula for molality is m = moles of solute / kilograms of solvent. ... One formula we need to be aware of is the formula for density, which is d = m / v, where d is density, m is mass and v is volume.31-Oct-2021
How do you convert molarity to molality and density?
The conversion requires multiplying the molarity by the solution's volume to get the number of moles of solute, then dividing by the solution's mass to get molality. Of course you can usually simplify this further by noting that D=m/V, so molality is equal to the molarity divided by the solution's density.
How do you find mass with molarity and density?
For example, if the density of 0.5 M KCl solution is 1.1 g/ml, the weight of 1 liter of the solution is 1.1 x 1,000 = 1,100 g. Divide the mass of the dissolved compound by the mass of the solution, and multiply the result by 100 to calculate percentage.26-Apr-2018
Why is molarity preferred over molarity?
Molarity is number of moles per unit volume of the solution and molality is number of moles per unit mass of solvent. Volume is temperature dependent where as mass is constant at all temperatures. So, molality remains constant but molarity changes with temperature. Hence , molality is preferred over molarity.25-Mar-2017
Is molarity and molality the same?
Molarity is the ratio of the moles of a solute to the total liters of a solution. The solution includes both the solute and the solvent. Molality, on the other hand, is the ratio of the moles of a solute to the kilograms of a solvent.29-Apr-2020
Is molarity the same as concentration?
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solution.
What is the relation between molarity and percent w w?
Originally Answered: what is the relationship bw molarity n weight percentage? Convert moles per litre to grams per litre by multiplying by Mr the molar mass. This is grams per 1000cm3. Divide by 10 to get % w/vol.
How do you find density using molarity?
Converting Molarity to Density If you have instead a 1.05 M NaCl solution, simply multiply the molarity by the molecular mass of NaCl to find the density in grams per liter: (1.05 * 58.44) = 61.32 g/l. Calculations are usually easier if you convert density to grams/milliliter by multiplying the result by 10-3.26-Apr-2018
What is the formula for molarity to molality?
Molarity = Moles Solute / Liter of Solution. Molality: The molality of a solution is calculated by taking the moles of solute and dividing by the kilograms of solvent. Molality is designated by a lower case "m".
How do you convert molarity to molarity?
Convert the expressions above to obtain a molarity formula. As mass / volume = molarity * molar mass , then mass / (volume * molar mass) = molarity . Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M .24-Nov-2021
How do you convert density to molar mass?
Molar mass is equal to density multiplied by molar volume.
What is the relation between molarity and density?
The relation between molarity (M) and molality (m) is given by: (ρ = density of solution (mg/mL), M1 = molecular weight of solute)
How do you find molality from density?
The formula for molality is m = moles of solute / kilograms of solvent. In problem solving involving molality, we sometimes need to use additional...
How do you convert molarity to molality when given density?
Conversion from Molarity to Molality. Problem: Find the molality of 18 M H2SO4. ... Make an assumption. Assume you have 1 L of solution. ... Find t...
How do you convert moles to molarity?
First you must calculate the number of moles in this solution, by rearranging the equation. No. Moles (mol) = Molarity (M) x Volume (L) = 0.5 x 2....
What is the difference between molarity and molality?
Of solvent in 1kg of solvent. The difference is that molarity is change with temperature but the molality does not depend on temperature. 16.2K views.
What is the molality of a substance?
Molality: It is defined as the moles of a substance/solute in 1 kg of a solvent. If 300 g of sugar (molar mass: 342 g/mol) is dissolved in 1500 g of water, the molality would be number of moles (342/300=0.87 moles) divided by 1500/1000 = 1.5 kg of solvent. Thus molality will be 0.87/1.5 = 0.58 moles/kg.
What is the preparation of a solution of a given molality?
The preparation of a solution of a given molality involves weighing both solute and solvent and getting their masses. But in the case of molarity, the volume of the solution is measured, which leaves room for variations in density as a result of the ambient condition of temperature and pressure.
What is the mole fraction of water?
It is equal to the moles of one component divided by the total moles in the solution or mixture. For example, if 3.47 moles of water and 8.54 moles of acetone are mixed together, the mole fraction of water will be 3.47 divided by the total moles (which is 12.01), and is therefore 0.289. Related Answer. Ruben Valenzuela.
How many units are in a mole?
Just like a dozen refers to 12 units, a mole refers to 6.022 x10^23 units! One can also say that 12g is the weight one mole of C atoms. (Total number of atoms per mole) multiplied by the (weight of each atom) = Weight per mole. This weight is referred to as the ‘formula mass’ of an element/molecule.
Relationship between density and molarity
Today in discussion we learned that density was related to molarity as they had similar equations (D = m/V and C = n/V) and were given the example: 6.54g (1cm^3/0.7857) = 8.32 cm^3 = 8.32 mL While I understand the calculations and the relationship between cm^3 and mL, I'm not sure where the 6.54 or 0.7857 comes from since there was no other context provided, as far as I could tell.
Re: Relationship between density and molarity
I'm assuming that you were given the mass of a substance (6.54 grams) and the density of the substance (0.7857 g/cm^3). Since the equation was D= m/V he substituted in D and m to find volume. I think he was trying to say like the C =n/V problems, you can find any variable of the D = m/V equation as long as you have two given variables.
Re: Relationship between density and molarity
I believe the 6.54g is the mass of the substance, then you divide it by (0.7857/1cm^3) because that is equal to 0.7857/1mL which is the density which is mass/volume.