| PubChem CID | 25353 |
|---|---|
| Structure | Find Similar Structures |
| Chemical Safety | Laboratory Chemical Safety Summary (LCSS) Datasheet |
| Molecular Formula | Cl2S |
| Synonyms | SULFUR DICHLORIDE Dichlorosulfane 10545-99-0 Sulphur dichloride Sulfur chloride (SCl2) More... |
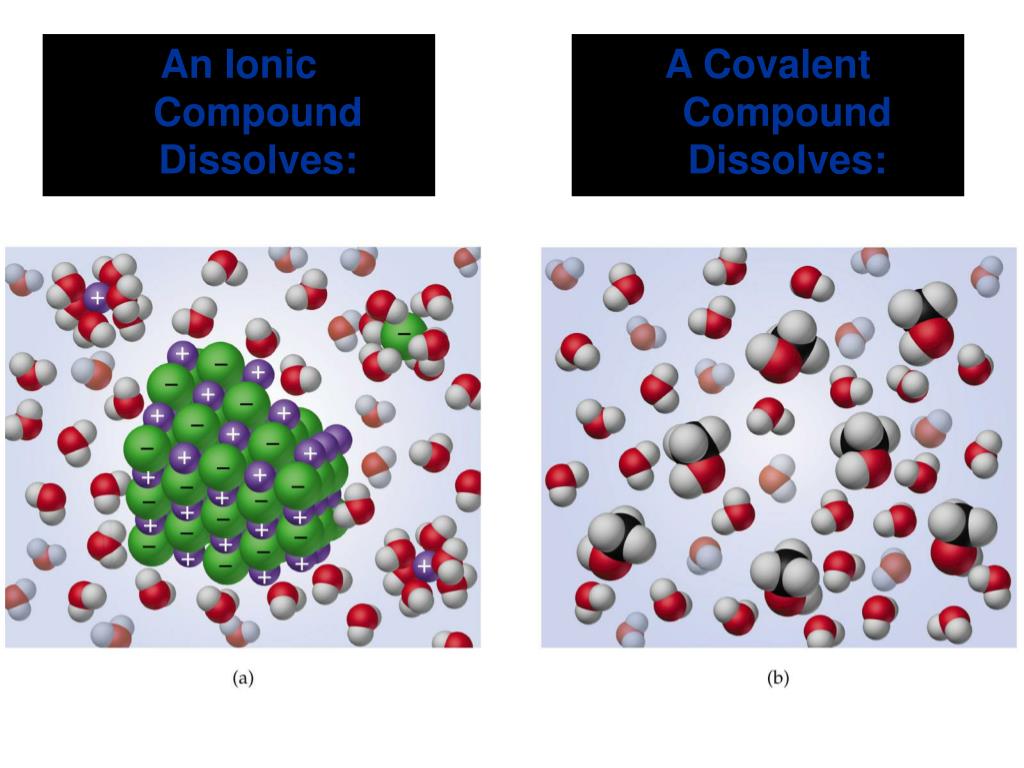
Does SCl2 contain ionic compound?
Sodium chloride is an ionic compound. Many bonds can be covalent in one situation and ionic in another. For instance, hydrogen chloride, HCl, is a gas in which the hydrogen and chlorine are covalently bound, but if HCl is bubbled into water, it ionizes completely to give the H+ and Cl- of a hydrochloric acid solution.
What's the difference between ionic and covalent?
Ionic vs Covalent Bonds Summary
| Ionic Bonds | Covalent Bonds | |
| Description | Bond between metal and nonmetal. The non ... | Bond between two nonmetals with similar ... |
| Polarity | ||
| Shape | No definite shape | Definite shape |
| Melting Point | High | Low |
Is SOCl2 ionic or covalent?
socl2 ionic or covalent - artisanfellow.com ... Polar
What are 5 examples of ionic compounds?
Ionic bond examples include:
- LiF - Lithium Fluoride
- LiCl - Lithium Chloride
- LiBr - Lithium Bromide
- LiI - Lithium Iodide
- NaF - Sodium Fluoride
- NaCl - Sodium Chloride
- NaBr - Sodium Bromide
- NaI - Sodium Iodide
- KF - Potassium Fluoride
- KCl - Potassium Chloride
Does SCl2 have a covalent bond?
It is covalent (molecular).Feb 21, 2018
What is the name for covalent compound?
Binary molecular (covalent) compounds are formed as the result of a reaction between two nonmetals....Binary molecular (covalent) compounds.compoundsystematic namecommon nameNOnitrogen monoxidenitric oxideNO2nitrogen dioxideN2O3dinitrogen trioxideN2O4dinitrogen tetroxide2 more rows
What is the name of the covalent compound of2?
Oxygen difluoridePubChem CID24547StructureFind Similar StructuresChemical SafetyLaboratory Chemical Safety Summary (LCSS) DatasheetMolecular FormulaF2O or OF2SynonymsOxygen difluoride Difluorine monoxide FLUORINE MONOXIDE Oxydifluoride Fluorine oxide More...3 more rows
What is the name of the covalent compound SiO2?
SiO2 is the chemical compound silicon dioxide. It is formed when silicon is exposed to oxygen. It has a covalent bond and is a superior electric insulator, posessing high chemical stability. Quartz is the second most common mineral in the Earth's continental crust.
SCl2 Valence Electrons
Sulfur is in group 6 (Chalcogens) of the periodic table with the electronic configuration [Ne] 3s²3p⁴. Therefore, the Sulfur atom contributes 6 x 1 = 6 valence electrons Being in group 7 of the periodic table, Chlorine has seven valence electrons with a valency of -1. Chlorine’s electronic configuration is given by [Ne]3s23p5.
SCl2 Lewis Structure
Sulfur is the least electronegative and therefore, is placed in the center of the skeletal structure. We then line up the two Chlorine atoms on either side of the Sulfur atom. We will now begin placing the 20 valence electrons available to us in accordance with the octet rule.
SCl2 Hybridization
Sulfur Dichloride comprises two Chlorine atoms separated by a lone Sulfur atom. This Sulfur atom forms two covalent bonds with each of the Chlorine atoms. The fulfillment of the octet rule on each of the Chlorine atoms leaves 4 valence electrons. These act as lone pairs and attach themselves on opposite ends of the Sulfur atom.
SCl2 Bond Angles
According to the VSEPR theory, the Chlorine atoms and the lone pairs repel each other. This gives SCl2 a bond angle of 103°.
SCl2 Molecular Geometry and Shape
As seen in the Lewis structure above, the Chlorine atoms repel each other. This gives us a linear shape initially. However, upon the addition of the two lone pairs on Sulfur, the molecular geometry becomes bent. This is in accordance with the VSEPR theory.