How to write the name for Na3N?
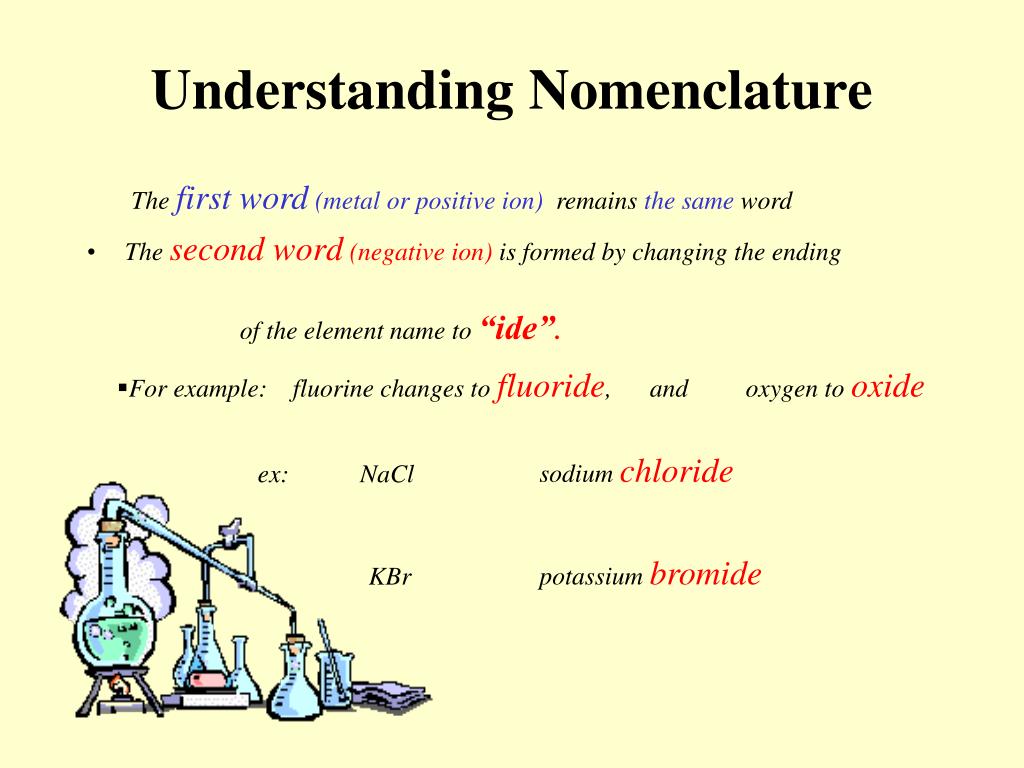
Learning to name chemical compounds requires that you:
- Determine the type of compound you are working with.
- Apply the rules for naming that type of compound.
- Practice until it becomes second nature.
What is the compound name for NaNO3?
What are examples of non binary compounds?
- NaNo3. Sodium Nitrate.
- Ca (OH)2. Carbon Hydroxide.
- K2CO3. Potassium Carbonate.
- NH4Cl. Ammonium Chloride.
- MgSO4. Magnesium Sulfate.
- AlPO4. Aluminum Phosphate.
- (NH4)2SO4. Ammonium Sulfate.
- Na3PO4. Sodium Phosphate.
What are the rules for naming compounds?
- Alkanes - saturated hydrocarbons The names of the straight chain saturated hydrocarbons for up to a 12 carbon chain are shown below. ...
- Alkyl halides The halogen is treated as a substituent on an alkane chain. ...
- Alkenes and Alkynes - unsaturated hydrocarbons Double bonds in hydrocarbons are indicated by replacing the suffix -ane with -ene . ...
What are the systematic names of these compounds?
Sometimes these compounds have generic or common names (e.g., H2O is "water") and they also have systematic names (e.g., H2O, dihydrogen monoxide). The common name must be memorized. The systematic name is more complicated but it has the advantage that the formula of the compound can be deduced from the name.
What element is Na3N?
Sodium NitrideSodium Nitride is an inorganic compound with the chemical formula Na3N. It can be generated by combining the elements of Sodium and Nitrogen settled on the low-temperature sapphire substrate.
Why is sodium nitride Na3N?
In ionic compounds, the charges of constituent ions must balance. This can be achieved by having three sodium ions per nitride ion. Therefore, the formula of sodium nitride is Na3N .
What is the name of alcl3?
aluminium chlorideAluminium chloride / IUPAC ID
Is Na3N a salt?
. This alkali metal nitrate salt is also known as Chile saltpeter (large deposits of which were historically mined in Chile) to distinguish it from ordinary saltpeter, potassium nitrate....Sodium nitrate.NamesChemSpider22688ECHA InfoCard100.028.686EC Number231-554-3E numberE251 (preservatives)50 more rows
What type of bond is Na3N?
ionic bondThis is how the ionic bond forms in Sodium Nitride (Na3N).
How is Na3N formed?
It is an inorganic compound which is a highly unstable alkali metal nitride. It is obtained by uniting atomic beams of nitrogen and sodium deposited on a low-temperature substrate of sapphire. It has the ability to readily decompose into its elements. The molecular or chemical formula of Sodium Nitride is Na3N.
Why is it called aluminum chloride?
However, aluminum chloride, AlCl3, is sometimes called aluminum trichloride which is not incorrect in this case because it actually is a molecular compound (it has very polar aluminum-chlorine covalent bonds) even though it looks like it should be ionic since it contains metal and nonmetal elements typical of ionic ...
Why Aluminium chloride is AlCl3?
What is Aluminium Chloride (AlCl3)? Aluminium chloride is also sometimes referred to as aluminium trichloride or aluminium (III) chloride. The compound is formed when aluminium and chlorine are reacted together. Its chemical formula is written as AlCl3.
Is AlCl3 a salt?
A salt is an ionic compound produced by reaction of an acid with a base. AlCl3 is formed by reaction of Al(OH)3 and HCl. Therefore, it is a salt.
What does saltpeter look like?
Pure saltpeter or potassium nitrate is a white crystalline solid, usually encountered as a powder.
What is E250?
Sodium nitrite (food additive E250) is used in the food industry as a color fixer and preservative clamp in meat and fish products. Chemical formula of sodium nitrite is: NaNO2. In pure form the additive E250 represents white hygroscopic crystal powder with slightly yellowish shade.
What is nitrate salt?
Salts of nitric acid containing the group NO3- are called nitrate salts. Nitrate anions form salts with a wide range of elements in the periodic table. Examples of nitrates are ammonium nitrate (NH4NO3), sodium nitrate (NaNO3) and potassium nitrate (KNO3).
What is the chemical formula for sodium nitride?
Chemical compound. Sodium nitride is the inorganic compound with the chemical formula Na 3 N. In contrast to lithium nitride and some other nitrides, sodium nitride is an extremely unstable alkali metal nitride.
What color is sodium nitride?
Sodium nitride can be of reddish brown or dark blue color depending on the synthesis of the compound due to intrinsic properties. It shows no signs of decomposition after several weeks when at room temperature. The compound does not have a melting point as it decomposes back into its elemental forms as demonstrated using mass spectrometry around 360 K. The estimated enthalpy of formation for the compound is +64 kJ/mol.
How is sodium nitride synthesized?
Sodium nitride can be synthesized in two different ways: by the thermal decomposition of NaNH 2 or by the direct reaction of the elements. The most common way to successfully synthesize sodium nitride has been done by Dieter Fischer & Martin Jansen and Grigori Vajenine using the latter method.
How to name a compound?
Learning to name chemical compounds requires that you: 1 Determine the type of compound you are working with. 2 Apply the rules for naming that type of compound. 3 Practice until it becomes second nature.
Is Na3N a metal?
Hint for Naming Na3N. Sodium (Na) is a metal. Nitrogen (N) is a non-metal. This is a binary ionic compound. First write the name for Na as it is written on the Periodic Table. Next for the non-metal (N) use the name on the Periodic Table (Nitr ogen )but replace the ending ogen with ide . Show Answer.
The framework of Sodium Nitride Formula
The Anti-ReO structure of sodium nitride is seen in its formula. A straightforward lattice framework is additionally located in sodium nitrite, which is composed of NNa Octahedra. Around 236.6 pm is the length of the N-Na bond. X-ray diffraction utilizes to record the structural formation of sodium nitrite.
Structure of Sodium Nitride
By thermal decomposition of NaNH2 or by the straightforward approach, sodium nitrite manufacture. Dental caries produce when sodium forms in nitrogen, and nitrogen introduce right into it as sodium ions, and also, they will undoubtedly rearrange themselves and fit near nitrogen.