How many bonds can unhybridized carbon make?
What is the angle of a SP2 orbital?
How to draw Lewis structure?
What is the formal charge of a bond?
What is the Valence Shell Electron Repulsion Theory?
What is the directional arrangement of covalent bonds?
How many electrons are needed to form a formaldehyde bond?
See more
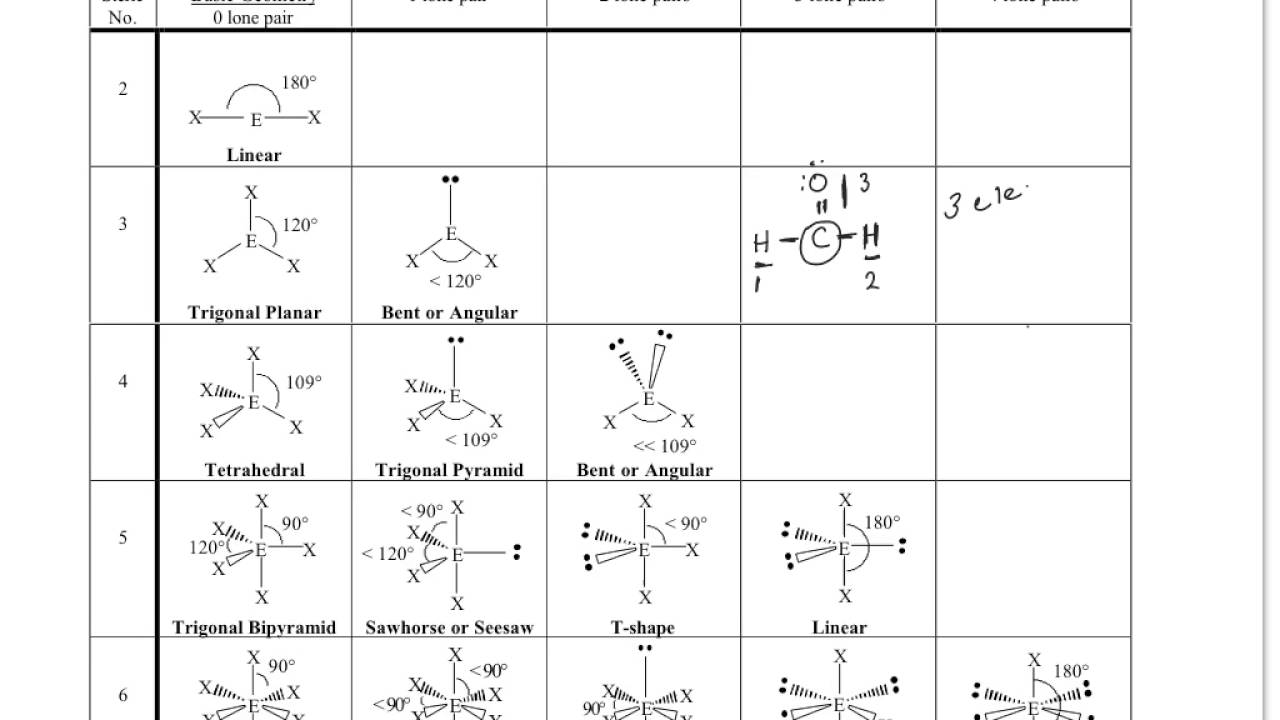
What molecular shape is H2CO?
H2CO lewis structure, molecular geometry, polarity, bond angle, hybridizationName of MoleculeFormaldehydeChemical formulaH2COMolecular geometry of H2COTrigonal planarElectron geometry of H2COTrigonal planarHybridizationSp22 more rows•Mar 17, 2022
What is the molecular structure of CH2O?
Formaldehyde (CH2O) lewis dot structure, molecular geometry, polar or non-polar, hybridizationName of MoleculeFormaldehydeChemical formulaCH2OMolecular geometry of CH2OTrigonal planarElectron geometry of CH2OTrigonal planarHybridizationSp22 more rows
What is Lewis structure of H2CO?
0:001:33How to Draw the Lewis Structure for H2CO - YouTubeYouTubeStart of suggested clipEnd of suggested clipThis is the h2 CEO Lewis structure. We have a total of 12 valence electrons for this h2 co2 aMoreThis is the h2 CEO Lewis structure. We have a total of 12 valence electrons for this h2 co2 a structure will start by putting carbon in the middle and then hydrogen's always go on the outside.
Is H2CO polar or nonpolar molecule?
0:011:35Is H2CO Polar of Non-Polar? - YouTubeYouTubeStart of suggested clipEnd of suggested clipLet's take a look at whether h2co is polar or nonpolar this is formaldehyde. So we'll start with ourMoreLet's take a look at whether h2co is polar or nonpolar this is formaldehyde. So we'll start with our lewis structure here and we want to look at the bonds. So if we look at the carbon here and the
What is ph3 Lewis structure?
0:001:02How to Draw the Lewis Structure for PH3 - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe have three of them. We'll put two electrons between each of the atoms to form chemical bonds we'MoreWe have three of them. We'll put two electrons between each of the atoms to form chemical bonds we've used six valence electrons. Put the last two in the center for a total of 8 valence electron.
What H2CO 3?
1:342:43Lewis Structure (+VSEPR) for H2CO - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo this is my lewis structure we've got a single bonded hydrogen single bonded hydrogen and doubleMoreSo this is my lewis structure we've got a single bonded hydrogen single bonded hydrogen and double bonded oxygen if you're into Vesper you'll recognize this as a x3 because the central atom has three
What is the lewis structure for ch3br?
0:000:51How to Draw the Lewis Structure for CH3Br (Bromomethane) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe hydrogen's each have two the bromine has eight and the carbon has 8 as well so the outer shellsMoreThe hydrogen's each have two the bromine has eight and the carbon has 8 as well so the outer shells are full on all the atoms in ch3 BR. So that's it this is the lewis structure for CH 3br.
Why is H2CO trigonal planar?
0:201:59H2CO (Formaldehyde) Molecular Geometry, Bond Angles (and ...YouTubeStart of suggested clipEnd of suggested clipSo we have three things attached. And we don't have any lone pairs on the carbon. These electronsMoreSo we have three things attached. And we don't have any lone pairs on the carbon. These electrons here they're all involved in chemical bonds. So zero lone pairs it's trigonal planar and the bond
What intermolecular forces are present in H2CO?
H2CO is a polar molecule and will have both dipole-dipole forces and London dispersion forces while CH3CH3 is a non-polar molecule and will only have London dispersions forces.
What is the shape of Ch2Cl2?
The shape for ch2cl2 is tetrahedral. The lewis structure drawing is really misleading because it makes you think that the chlorine atoms are directly opposite from each other on a 1D surface.
Is a carbon atom a trigonal planar molecule?
It's a trigonal planar molecule because the carbon atom is sp2 hybridizated and forms 3 bonds. The atoms attached to it are 2 hydrogen atoms (single bond) and one oxygen (double bond).
How many bonds can unhybridized carbon make?
Unhybridized carbon will only be able to make 2 single bonds along the internuclear axis (usually the z-axis), using s orbital and one pz orbital. But there are three single bonds in Formaldehyde so hybridization becomes important. The sp2 hybrid orbitals are planar with an angle of 120⁰.
What is the angle of a SP2 orbital?
The sp2 hybrid orbitals are planar with an angle of 120⁰. The unhybridized 2p orbital on carbon is perpendicular to the molecular plane. It is used for sideways overlap to form a π bond with the 2p orbital of oxygen.
How to draw Lewis structure?
Step 1: Calculate total no. of valence atoms in molecule i.e. the group no. of every atom plus total negative charge or minus the total positive charge. Formaldehyde is a neutral molecule so it has zero net charges. Step 2: Choose the central atom.
What is the formal charge of a bond?
Formal charge=No. of valence electrons - 12*No. of bonding electrons-No. of lone pairs
What is the Valence Shell Electron Repulsion Theory?
The Valence Shell Electron Repulsion Theory attempts to predict the geometry of individual molecules using the concept of minimum energy and maximum stability.
What is the directional arrangement of covalent bonds?
Covalent bonds are directional which means they have a specific arrangement in space. Hybridization helps us understand the nature of these covalent bonds using atomic orbitals of the central atom. In the case of formaldehyde, let’s take carbon. Carbon has an electronic ground state configuration of 1s2 2s2 2p2.
How many electrons are needed to form a formaldehyde bond?
It is a good idea to start with the most negative elements first while adding lone pair of electrons. We have formed three bonds using 6 electrons for formaldehyde. That leaves 6 electrons, all of which are used upon oxygen. Step 4: We have to complete the octet on the central atom.