What is the average atomic mass for tin?
Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure. The chemical symbol for Tin is Sn. Atomic mass of Tin is 118.71 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
What is the formula to find molar mass?
What is the formula to find molar mass? The characteristic molar mass of an element is simply the atomic mass in g/mol. However, molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant (1 g/mol).To calculate the molar mass of a compound with multiple atoms, sum all the atomic mass of the constituent atoms.
How to find the molar mass of an unknown element?
Method 2 Method 2 of 2: Calculating the Molar Mass of a Compound
- Find the chemical formula for the compound. This is the number of atoms in each element that makes up the compound.
- Find the relative atomic mass of each element in the compound. Using the periodic table, locate the relative atomic mass for each element.
- Calculate the molar mass of each element in the compound. ...
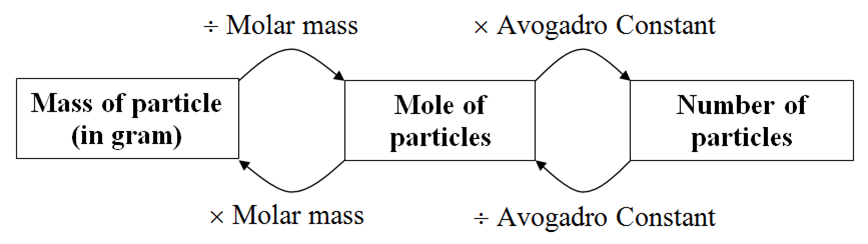
What is the formula for converting mass to moles?
Convert the expressions above to obtain a molarity formula. As mass / volume = molarity * molar mass, then mass / (volume * molar mass) = molarity. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass.
What is the mole of tin?
Tin has a molar mass of 118.71g/mol . So, there will be around 2.1 moles of tin atoms.
What is the molar mass of tin?
118.71 uTin / Atomic mass
What is the mass of 1 moles?
The mole is related to the mass of an element in the following way: one mole of carbon-12 atoms has 6.02214076 × 1023 atoms and a mass of 12 grams.
What is the mass of Sn in grams?
The Elements, sorted by Atomic MassAtomic NumberSymbolAtomic Weight (amu, g/mol)47Ag107.86848Cd112.4149In114.8250Sn118.6971 more rows
What is the mass in grams of 1 tin atom?
118.71 uWhat is the average mass of a tin atom in grams? The atomic mass of tin is 118.71 u.
How do I calculate moles?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
How do u calculate mass?
One way to calculate mass: Mass = volume × density. Weight is the measure of the gravitational force acting on a mass.
What is the mass of 1 mole of water?
18.01528 g/molWater / Molar mass
How do you find the mass of 1 mole of any element?
Avogadro's number is the number of particles in one mole of anything. In this context, it is the number of atoms in one mole of an element. It's easy to find the mass of a single atom using Avogadro's number. Simply divide the relative atomic mass of the element by Avogadro's number to get the answer in grams.
How do you convert mass to moles?
0:5213:17How To Convert Grams To Moles - VERY EASY! - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo here we need to divide. It's going to be 30 divided by 12 which is 2.5. So 30 grams of carbon isMoreSo here we need to divide. It's going to be 30 divided by 12 which is 2.5. So 30 grams of carbon is equivalent to 2.5 moles of carbon.
What is molar mass of Sn in g mol?
118.710 gStandard atomic weights usedv t e Chem molar mass testsAtomic numberElementMolar mass50tin118.710 g·mol−151antimony121.760 g·mol−152tellurium127.60 g·mol−175 more rows
How do I calculate molar mass?
0:223:51How to Calculate Molar Mass (Molecular Weight) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we go to the periodic. Table we find carbon and we find oxygen and we see the atomic mass thereMoreSo we go to the periodic. Table we find carbon and we find oxygen and we see the atomic mass there right below the element symbol to find the molar mass we add those two numbers together.
How can molar mass be calculated?
0:133:51How to Calculate Molar Mass (Molecular Weight) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we go to the periodic. Table we find carbon and we find oxygen and we see the atomic mass thereMoreSo we go to the periodic. Table we find carbon and we find oxygen and we see the atomic mass there right below the element symbol to find the molar mass we add those two numbers together.
What is the volume of tin?
The volume of a cylindrical tin can, V=16π inches3 V = 16 π i n c h e s 3 . Here, the volume formula of the cylindrical can is, V=πr2h V = π r 2 h .
What is the symbol of tin?
SnTin / Symbol
How many neutrons are in tin?
69 neutronsThis tin atom has 50 protons, 69 neutrons and 48 electrons.
Computing molar mass (molar weight)
To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Computing molecular weight (molecular mass)
To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
Definitions of molecular mass, molecular weight, molar mass and molar weight
Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12)
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
How to find the formula weight?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
How many grams are in a mole of tin?
The molecular formula for Tin is Sn. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Tin, or 118.71 grams.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
Atomic Number of Tin
Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure. The chemical symbol for Tin is Sn.
Atomic Mass of Tin
Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.