| Property Name | Property Value |
|---|---|
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 87.99361265 |
| Monoisotopic Mass | 87.99361265 |
Does carbon have a greater molar mass then chlorine?
The mass of 1 mole of magnesium is greater than the mass of 1 mole of carbon. A compound has a molar mass of 180.15g/mol. Given the following percent composition calculate the molecular formula: 40% C, 6.7%H, 53.3% O
Is carbon tetrafluoride a polar molecule?
Question = Is carbon tetrafluoride polar or nonpolar ? Answer = carbon tetrafluoride ( CF4 ) is Nonpolar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
How to convert atoms of carbon to moles of carbon?
Key Points
- Avogadro’s number is a very important relationship to remember: 1 mole = 6.022×1023 6.022 × 10 23 atoms, molecules, protons, etc.
- To convert from moles to atoms, multiply the molar amount by Avogadro’s number.
- To convert from atoms to moles, divide the atom amount by Avogadro’s number (or multiply by its reciprocal).
What is the formula of carbon tetrahydride?
The formula for carbon tetrahydride is CH4. Some other names for this compound are methane and marsh gas. Methane is a hydrocarbon that belongs to the paraffin series. It is an odorless, colorless gas. Methane's melting point is negative 296.5 degrees Fahrenheit, and its boiling point is negative 259.6 degrees Fahrenheit.
How do you calculate the mass of a molecule in grams?
You look up the atomic mass of each of the elements in the formula, multiply it by the number of atoms of that element in the compound and add it to all the others. This gives you the mass, in grams, of one mole of the molecule.Apr 26, 2018
What is carbons mass in grams?
The mass of a single carbon atom is 1.994 x 10-23 g. The mass of a single atom is an extremely small number! This is why chemists use Avogadro's number. It makes working with atoms easier because we work with moles rather than individual atoms.Jun 2, 2021
What is the mass of molecules co2 in grams?
44.01gThe molecular mass of carbon dioxide is 44.01amu. The molar mass of any compound is the mass in grams of one mole of that compound. One mole of carbon dioxide molecules has a mass of 44.01g, while one mole of sodium sulfide formula units has a mass of 78.04g. The molar masses are 44.01g/mol and 78.04g/mol respectively.Feb 21, 2022
How do you find the mass of carbon?
To calculate the mass of a single atom of carbon, we just need to divide the molar mass of 12.0 g (0,012 kg) by the number of particles per mole (Avogadro's number). Doing so yields 1.99 ´ 10–26 kg as the mass of a carbon atom.
How do I calculate molecular mass?
Molecular Mass Problem Find the atomic mass for each element by using the mass given in the Periodic Table. Multiply the subscript (number of atoms) times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass.Mar 11, 2019
What is mass of carbon in carbon dioxide?
-We know that the atomic weight of carbon is 12 grams and that of oxygen is 16 grams. Hence we have calculated the mass 5 of carbon in carbon dioxide to be 27.27%.
How do you find the mass of carbon in CO2?
To find the mass of carbon present in the CO2 in grams, we need only to multiply the moles of carbon by the molar mass of carbon. This 7.4 × 1015 g of carbon is 7.4 billion metric tons of carbon that are generated each year. This mass amount of carbon is less than the mass amount of carbon dioxide.
What is mass of a molecule of CO2?
about 44gMass of 1 mole (6.023 X 1023 molecules) of CO2 is about 44g.
How to calculate molecular mass?
Molecular mass (older name molecular weight) is the mass of a molecule calculated as the sum of the mass of each atom in the molecule multiplied by the number of atoms of that element in the molecule. Molecular mass is a dimensionless quantity numerically equal to the molar mass. Though molecular and atomic mass values are dimensionless, they are given the unit dalton (Da) or unified atomic mass unit (u), which is approximately the mass of a single proton or neutron and is numerically equivalent to 1 g/mol.
What is the unit of mass?
The molar mass is a physical property, which is defined as the mass of a substance divided by its amount of substance in moles. In other words, it is the mass of one mole of a particular substance. In SI, the unit for molar mass is kg/mol. However, chemists almost always express molar masses in g/mol for convenience.
What is the mole of a substance?
All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions. The mole, symbol mol, is the SI unit of the amount of substance. One mole contains exactly 6.02214076×10²³ elementary entities. This number is the fixed numerical value of the Avogadro constant, N A, when expressed in the unit mol⁻¹ and is called the Avogadro number. The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles.
Molar mass
The total sum of the masses of the atoms or elements present in the molecule is termed as molecular mass.
Molar mass of Carbon tetrachloride
Carbon tetrachloride comprises of one carbon atom and four chlorine atoms.
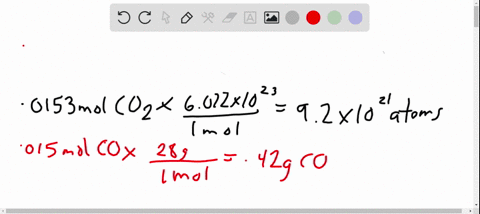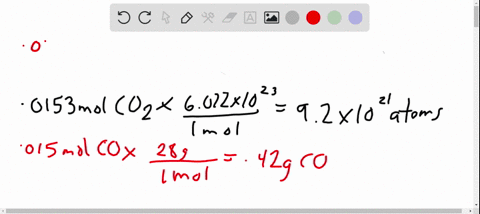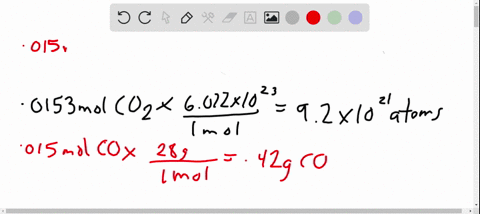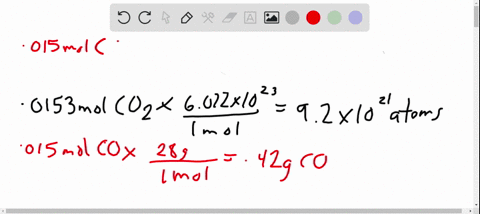
Mole
Molar Mass
- The molar mass is a physical property, which is defined as the mass of a substance divided by its amount of substance in moles. In other words, it is the mass of one mole of a particular substance. In SI, the unit for molar mass is kg/mol. However, chemists almost always express molar masses in g/mol for convenience. Molar mass = grams/mole
Molar Masses of Elements and Compounds
- Compounds are substances consisting of several different atoms held together by chemical bonds. For example, the following substances that can be found in every kitchen are compounds: 1. salt (sodium chloride) NaCl 2. sugar (sucrose) C₁₂H₂₂O₁₁ 3. vinegar (acetic acid solution) CH₃COOH The molar mass of elements in grams per mole is numerically equal to their atomic m…
Molecular Mass
- Molecular mass (older name molecular weight) is the mass of a molecule calculated as the sum of the mass of each atom in the molecule multiplied by the number of atoms of that element in the molecule. Molecular mass is a dimensionlessquantity numerically equal to the molar mass. Though molecular and atomic mass values are dimensionless, they are given the unit dalton (Da…
Calculating The Molar Mass
- The molar mass of a substance is calculated using three steps: 1. Finding the atomic masses of elements in the periodic table. 2. Counting the number of atoms of each element in the compound. 3. Finding the molar mass by means of calculating the sum of the atomic weight of the atoms, which form the compound multiplied by their numbers. For example, let us calculate t…