How to determine the number of moles in HCl?
moles HCL = moles NaOH. We can calculate the number of moles of NaOH at the equivalence point by multiplying its concentration (in terms of molarity or moles/L) by the volume of NaOH added in order to reach the equivalence point: This tells us that there were 0.004398 moles of HCl in our original 0.02500 L solution.
How many grams are in 1 mole of HCl?
This compound is also known as Hydrochloric Acid. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles HCl, or 36.46094 grams. How many grams are in 1 mole of HCl?
What is the mass of 0.360 moles of HCl?
What is the mass of 0.360 moles of HCl? 13.1g. What is the mass of 0.118 moles of AgNO3? 20.0g. How many O atoms are present in 1.04 moles of MgSO4? 2.51×10^24 atoms. How many moles of silver ions are present in 32.46g of AgNO3? 0.191 moles. How many units of MgSO4 are present in 18.0g of this compound?
What is the molarity of 37% HCl?
The reagent used in laboratories is HCl dissolved in water, which is why you'll find in the label that it is around 37% HCl in weight. The other 63% is water. Considering 37% as the maximum solubility of HCl, you can calculate the molarity using the solution density (1.2 g/mL) and HCl's molar mass (36.46 g/mol).
What is the mass of one mole of HCl?
36.458 g/molHydrochloric acid / Molar mass
What is the mass of a HCl molecule?
0:000:33Molar Mass / Molecular Weight of HCl : Hydrochloric acid - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd we'll add those all up for a total of 36.46 grams per mole for the molecular weight of hcl. It'sMoreAnd we'll add those all up for a total of 36.46 grams per mole for the molecular weight of hcl. It's also called the molar mass of hcl.
How do you find moles of HCl?
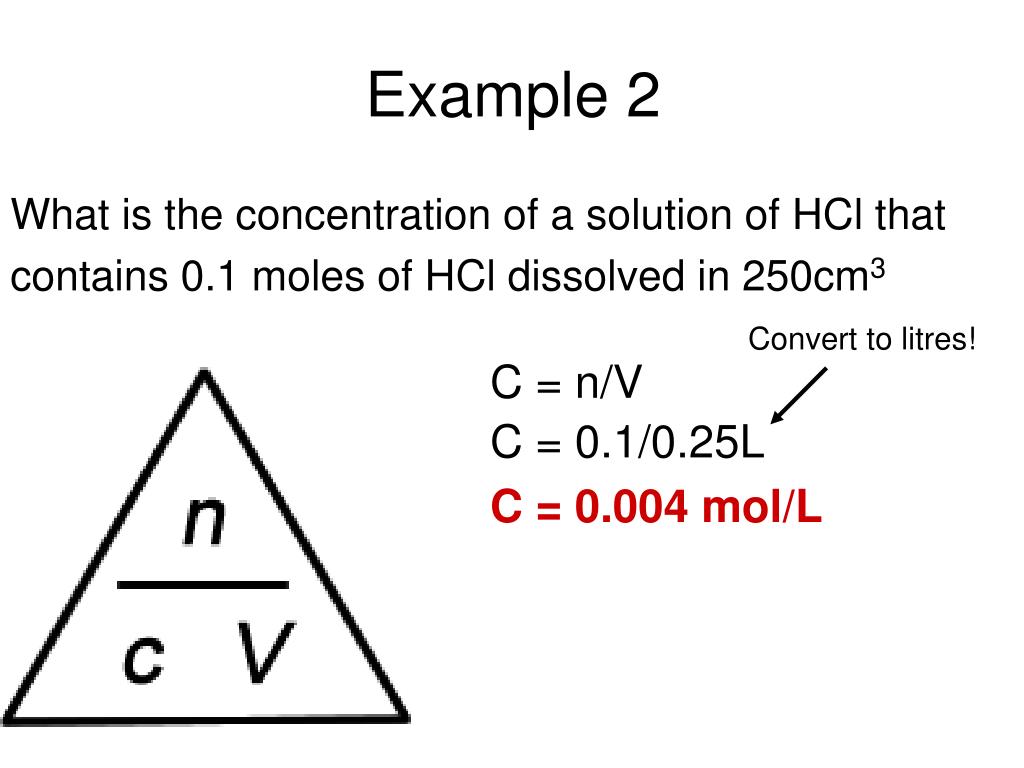
M = mol / L Calculate the molarity of an HCl solution which contains 18.23 g of HCl in 355.0 mL of solution. Calculate the number of moles of HCl. Divide the number of moles of HCl by the total volume in liters.
What is the mass of hydrogen atom in 1 mole HCl *?
The mass of one mole of a hydrogen atom is 1 gram, whereas the mass of one mole of hydrogen molecules is 2 grams. One mole of hydrogen molecule has a mass of 2 grams because hydrogen molecules are formed by binding two hydrogen atoms.
How many moles are in HCl?
Using periodic table you can find molar mass of HCl, which is 36.458 g/mol. This means 1 mol of HCl is 36.458 grams. So 20 g of HCl is 0.548 mol.
How do you make 1M HCl?
1M HCl: add 1mol/12M = 83 ml conc. HCl to 1L of water or 8.3ml to 100ml.2M HCl: add 2mol/12M = 167 ml conc. HCl to 1L of water or 16.7ml to 100ml.
What is the meaning of 1 mole HCl?
A 1 molar (M) solution will contain 1.0 GMW of a substance dissolved in water to make 1 liter of final solution. Hence, a 1M solution of NaCl contains 58.44 g. Example: HCl is frequently used in enzyme histochemistry.
How do you find the mass of HCl?
Molar mass of Hydrochloric acid = Molar mass of H + Molar mass of Cl = 1 + 35.5 = 36.5 g.
What is the mass of 2 moles of HCl?
2 moles HCl to grams = 72.92188 grams.
How do you find the mass of 1 mole?
0:021:011.2.2 Calculate the mass of one mole of a species from its formula.YouTubeStart of suggested clipEnd of suggested clipThe relative atomic mass is thirty five point four five and that equals a molar mass of fifty eightMoreThe relative atomic mass is thirty five point four five and that equals a molar mass of fifty eight point four four grams per mole.
What is the mass in grams of HCl?
Molar mass of Hydrochloric acid = Molar mass of H + Molar mass of Cl = 1 + 35.5 = 36.5 g.
What is the equivalent mass of HCl?
85.1gNow equivalent mass is given as the molecular weight divided by the n-factor. Therefore, the equivalent mass of HCl is 85.1g.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight ...
How much does HCl have in its mass?
The mass of one H atom is 1amu ,and the mass of one Cl atom is 35.5amu , so mass of HCl is 1+35.5=36.5amu
What is the mass of Cl?
It depends on which isotope of Cl one is using. That’s why the mass of Cl is usually stated as 35+some decimal. That value is used when one is working on a macro scale. If one is speaking of a single molecule of HCl, the mass will be a whole number of amu.
How many grams are in a mole of chlorine?
This is the weighted average mass of chlorine, including its isotopes, as found in nature. This also means that one mole of chlorine atoms has a mass of 35.453 grams. A mole is just a name for a certain number, much like a dozen is just a name for 12. A mole of something means that you have 6.023 x 10^23 units of that something, just like a dozen of something means that you have 12 units of that something. So if you have a mole of Cl atoms you have 6.023 x 10^23 atoms of Cl. Since a mole of chlorine atoms has a mass of 35.453 grams, then a weighted average mass for one of those atoms will be equal to (35.453 grams/mole ÷ 6.023 x 10^23 atoms/mole =) 5.886 x 10^-23 grams per atom (notice the negative sign). That's a very small mass! It is 5.9 millionths, of a millionth, of a millionth, of a hundred-thousandth of a gram. To get a feel for how small that number is: Comparing the mass of a Cl atom to the mass of a 6-foot tall man is like comparing the mass of about 3/4 of a teaspoon of sugar to the mass of the entire earth!!
How to find the mass of a mol of electrons?
Just mutiply the mass of electrons by the Avogadro’s constant (roughly 6 x 10^23) and you will have the molar mass of electrons.
Which has more mass: an atom or a molecule?
Therefore a molecule will have more mass (mass of 3 apple together) than an atom (mass of 1 apple) .