Chloroform (CHCl3) lewis dot structure, molecular geometry, hybridization
| Name of Molecule | Chloroform |
| Chemical formula | CHCl3 |
| Molecular geometry of CHCl3 | Tetrahedral shape |
| Electron geometry of CHCl3 | Tetrahedral |
| Hybridization | Sp³ |
How do you write a Lewis dot diagram?
aim: how to write lewis dot structures (electron dot structures) do now: 1. read both sides of the handout. 2. write the electron configuration (orbital notation) of phosphorus atom, and phosphorus ion. 3. draw the lewis dot structure for the atom and the ion.
What are the Lewis dot structures?
Steps to Drawing a Lewis Structure
- Pick a central atom. Start your structure by picking a central atom and writing its element symbol. ...
- Count electrons. Lewis electron dot structures show the valence electrons for each atom. ...
- Place electrons around atoms. Once you have determined how many electrons to draw around each atom, you can begin placing them on the structure. ...
What is Lewis dot diagram?
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
What are some examples of Lewis structures?
Summary: Lewis Structures, VSEPR, and Molecular Polarity
- “Electron groups” include bonds, lone pairs, and odd (unpaired) electrons. A multiple bond (double bond or triple bond) counts as one electron group.
- A multiple bond (double bond or triple bond) counts as one bond in the VSEPR model.
- A = central atom, X = surrounding atoms, E = lone pairs
What is the structural formula for CHCl3?
CHCl₃Chloroform / Formula
When drawing the Lewis structure of the CHCl3 molecule the structure should represent a total of valence electrons?
In their outermost shells, carbon, chlorine, and hydrogen have four, seven, and one valence electrons, respectively. ∴ Total outermost valence shell electrons available for CHCl3 Lewis structure( dot structure) = 4+3*7+1*1=26 valence electrons in CHCl3.
What is the Lewis dot structure for ncl3?
0:002:42Lewis Structure of NCl3, Nitrogen Trichlorode - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo that's three sevens remember each chlorine brought seven with three of them that contributes 21MoreSo that's three sevens remember each chlorine brought seven with three of them that contributes 21 total plus an extra five is 26 valence electrons.
What is the electron domain of CHCl3?
Molecular Geometry Notation for CHCl3 Molecule :Name of MoleculeChloroform or trichloromethaneElectron geometry of CHCl3TetrahedralHybridization of CHCl3sp3Bond angle (Cl-C-H)109.5º degreeTotal Valence electron for CHCl3263 more rows
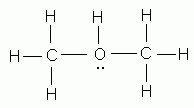
What is the Lewis dot structure for h2o?
0:001:21How to Draw the Lewis Structure for Water - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo 1 times 2 is 2 plus 6 2 plus 6 equals 8 we have a total of 8 valence electrons we'll put theMoreSo 1 times 2 is 2 plus 6 2 plus 6 equals 8 we have a total of 8 valence electrons we'll put the oxygen in the center and hydrogen's always go on the outside.
Is NCl3 linear?
0:051:37NCl3 Molecular Geometry / Shape and Bond Angles - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we have a lewis structure here for nitrogen trichloride. And we can see that the central nitrogenMoreSo we have a lewis structure here for nitrogen trichloride. And we can see that the central nitrogen here this is where we're looking at the molecular geometry. Has 1 2 3 and then the lone pair 4
What type of bond does NO2 have?
NO2 is a covalent compound. This is because the bond is formed between one nitrogen and two oxygen atom by the sharing of electrons.
What is the Lewis structure of n2?
0:002:29Lewis Structure of N2 (Nitrogen Gas) - YouTubeYouTubeStart of suggested clipEnd of suggested clipTwo nitrogen's each nitrogen according to the periodic table comes with five valence electrons boronMoreTwo nitrogen's each nitrogen according to the periodic table comes with five valence electrons boron comes with three carbon with four nitrogen. With five and there's two of them.
How many lone pairs of chlorine are there in CHCl 3 Lewis?
As well, there are no charges on atoms in CHCl 3 lewis structure.
How many electron pairs are there in CHCl 3?
After determining the center atom and sketch of CHCl 3 molecule, we can start to mark lone pairs on atoms. Remember that, there are total of thirteen electron pairs.
Why can't hydrogen be a center atom?
Hydrogen atom cannot be a center atom because hydrogen can only keep two electrons in last shell.
How to check the stability and minimize charges on atoms?
Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.
Why do we not need to reduce charges on atoms?
Because three are no charges on atoms, we do not need to do the step of reducing charges on atoms by converting lone pairs to bonds.
Is there a lone pair of carbon atoms?
Now, there are no more lone pairs to mark on carbon atom.
How many valence electrons are in C2H6?
This means that the Lewis dot structure for C2H6 must account for 14 valence electrons, either through bonding between atoms, or through lone pairs.
Is CHCl3 a solvent?
CHCl3 (trichloromethane, or chloroform) is a liquid at room temperature. It is a strong anesthetic and sedative, is used as a solvent, and is used extensively in organic synthesis. The Lewis structure helps understand how the valence electrons are involved in chemical bonding.
How many dots does each carbon atom have?
Each of carbon atoms will have 2 dots remaining for each of the other valence electrons. One of those will share a single dot (valence electron) from a hydrogen and the other a single dot (valence electron) from a chlorine atom. Don’t forget to draw the remaining 6 dots (lone pair valence electrons) around each chlorine atom to account for the full set of valence electrons.
How many electrons does a carbon atom share with a hydrogen atom?
The carbon atom shares each of its four outer electrons - three with chlorine and one with hydrogen. The chlorine atoms each share one of their 7 outer electrons with the carbon. The hydrogen atom shares its one electron with the carbon.
How many electrons are in a hydrogen atom?
If the number is more than eight then you’ve made a mistake. If the number is less than 8, then add in lone pairs sufficient to make the total up to eight. For hydrogen there should be two electrons.
How many dots are there on the Be?
Only two dots on the Be, since there are only two valence electrons.
Does NO2 have a Lewis structure?
There is actually another Lewis structure, one that puts the unpaired electron on oxygen. Molecules can actually have more than one Lewis structure at once: this is called resonance, and the truth is somewhere between all the Lewis structures. But the main or principal resonance structure for NO2 has the unpaired electron on nitrogen, not on one of the oxygens, because oxygen is more electronegative.
How many lone pairs are there in CHCl3 Lewis?
No lone pair is present on the central atom of the CHCl3 lewis structure. Each chlorine contains 3 lone pairs and is spaced evenly around the central atom.
Where is carbon in Lewis diagram?
Therefore place carbon at the center in the lewis diagram and chlorine or hydrogen spaced evenly around it.
How many electrons does hydrogen have?
But hydrogen can only hold a maximum of two electrons in its valence shell. So, placed these remaining valence electrons around the chlorine atom to complete their octet shell.
What is hybridization number?
Hybridization numbers can help to determine the hybridization of any atom or molecule. Generally, it is also called a steric number.
What is the valence electron of carbon?
As carbon belongs to the 14th periodic group, chlorine to the 17th, and hydrogen to the first group. Therefore the valence electron for carbon is 4, for chlorine 7, and for hydrogen, it is 1.
Which molecule is less electronegative?
Remember hydrogen always go outside in structure. Among carbon and chlorine, carbon is less electronegative because electronegativity increase from left to right in the periodic table.
Which molecule has no lone pair?
As we know overall shape of any molecule can be affected by the presence of lone pair on its central atom. According to the CHCl3 lewis structure, carbon is the central atom that has no lone pair on it.
How many valence shell electrons are in CHCl3?
For CHCl3, the total number of valence shell electrons on the central atom (C) is 4.
What is Lewis structure?
Lewis Structure is a simple representation of how valence shell electrons are arranged in a molecule. It tells us that a bond exists between atoms but does not tell us anything about the type of bonds.
How are valence electrons represented in Lewis structure?
In the lewis structure, valence electrons are represented by dots, and 2 bonding electrons between two atoms are represented by a line.
How many electrons are in the octet rule?
Octet Rule: Noble gases are considered stable, and they have 8 electrons in their valence shell.
Which atom is C?
Hence, C is the central atom in the chloroform atom.
Why is the central atom chosen?
The central atom is chosen so that it provides stability to the whole molecule, and electron density spread is facilitated.
What is the net charge of a molecule?
The net charge on the molecule is zero, but this does not guarantee that the formal charge on all atoms is also zero .
How many atoms are in CHCl3?
CHCl3 consists of a single Carbon atom, a single Hydrogen atom, and three Chlorine atoms.
What is the chemical formula for CHCl3?
The chemical formula CHCl3 represents Chloroform. It is also known as Trichloromethane. Chloroform is a clear, colorless liquid that possesses a pleasant odor. It is nonflammable and is denser than water. It is generally prepared by the chlorination of methane. Chloroform first found use as an inhalation anesthetic in the 19th century. These days, it is produced industrially as a precursor to making Teflon. Chloroform also finds use as a refrigerant in many parts of the world. This refrigerant- HCFC22, contributes to the depletion of the ozone layer. Chloroform also occurs naturally, with about 660,000 tons released per year from biotic and abiotic sources. It may also be released into the air due to Chlorination due to Chlorine’s presence in swimming pools and other water sources. It has the following properties:
How many covalent bonds are there in CHCl3?
Well, this one’s pretty simple. The CHCl3 molecule comprises three chlorine atoms and a Hydrogen atom; all pulled together by the central carbon atom. There are four covalent bonds present- 3 C-Cl bonds and 1 C-H bond. This structure gives rise to four electron domains. As such, the hybridization of the central Carbon atom is sp3.
How many valence electrons does chloroform have?
Chloroform or Trichloromethane comprises three Chlorine atoms, a Carbon atom and a hydrogen atom. Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s22p2. Therefore, the Carbon atom contributes 4 x 1 = 4 valence electrons Hydrogen has an electronic configuration of 1s1 and therefore contributes 1 valence electron. Being in group 7 of the periodic table, Chlorine has seven valence electrons with a valency of -1. Chlorine’s electronic configuration is given by [Ne]3s23p5. Therefore, the three Chlorine atoms contribute 7 x 3 = 21 valence electrons. Now, the total number of valence electrons available in CHCl3 is given by: 4 [C] + 1 [H] + 21 [Cl] = 26 valence electrons.
What is the bond angle of CHCl3?
CHCl3 has a Tetrahedral molecular structure and has bond angles of 109.5°.
Is CHCl3 a tetrahedral molecule?
With Hydrogen on the top and Chlorine atoms below, the molecular shape is shown in the figure. Therefore, CHCl3 has a Tetrahedral molecular geometr y . Concluding Remarks Let’s quickly summarize the salient features of CHCl3